Contributed by
B. D. Bruton
USDA ARS
Lane, OK 74555-0159
and
J. A. Duthie
Oklahoma State University
Plant Pathology Department
Lane, OK 74555-0128
Fusarium rot is one of the more common preharvest and postharvest diseases of cucurbit fruits.
Symptoms
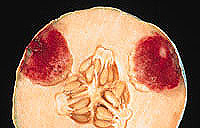
Symptoms of Fusarium fruit rot vary depending on the Fusarium species and the host. However, there are striking similarities in symptomatology among the Fusarium rots. F. graminum, F. acuminatum, F. culmorum, and F. moniliforme produce a distinct reddish or purplish pigmentation in the diseased area (Fig. 1). F. semitectum, F. equiseti, F. scirpi, and F. solani produce brown internal lesions; a cross section of a mature lesion reveals a dry, brown, spongy rot with a white halo (Fig. 2). There can be considerable variation in symptomatology depending on the stage of lesion development. In melon, lesions, which can be detected preharvest, generally remain green around the margin while the rest of the fruit begins to turn yellow at maturity. The disease is characterized by large fissures in epidermal tissue (Fig. 3). The net on the surface of the fruit is typically enlarged or thickened and is a dark tan. There is a distinct delineation between diseased and healthy tissue. Consequently, diseased tissue can be easily removed. High temperatures and high humidity encourage mycelial development after harvest. Symptoms produced by the purple-pigmented species are essentially the same, with the exception of the pigmentation. In melon, there is often no sign of infection prior to harvest, but numerous spongy white lesions may develop internally postharvest. This type of lesion normally does not produce the brown coloration internally.
|
|
|
 |
|
Fig. 1. Reddish pigmentation characteristic
of some Fusarium rot species (in this
example, Fusarium acuminatum)
on melon. Courtesy of B. D. Bruton. |
|
Fig. 2. Decayed internal tissue halo in
melon fruit. Courtesy of B. D. Bruton. |
|
 |
|
|
Fig. 3. Fissures in epidermal rind of melon. Courtesy of B. D. Bruton. |
|
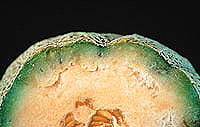
Fig. 4. Infection of melon rind by F. oxysporum f. sp. melonis. Courtesy of B. D. Bruton. |
|
Another Fusarium fruit rot occasionally encountered is caused by F. oxysporum f. sp. melonis. Unlike the other Fusarium spp., this fungus rarely penetrates the epidermis, but instead it invades the fruit through the stem end (Fig. 4), ultimately contaminating the seeds. There may be slight or no outward symptoms of fruit infection by F. oxysporum f. sp. melonis, and the internal symptoms are not characteristic of the other Fusarium rots. The most prominent symptom is a brown to purplish discoloration in the immediate area of the vascular bundles. The decay radiates out from the vascular bundles, and no pigmentation is imparted to the fruit. The decay is firm and may appear somewhat white because of
colonization by the fungus.
|
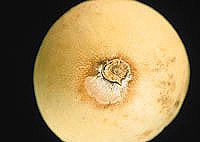
Fig. 5. Fusarium rot of honeydew. Courtesy of B. D. Bruton.
|
Cucumber, honeydew, squash, pumpkin, and watermelon are less frequently affected by the preharvest phase of Fusarium rot. In postharvest Fusarium rot of honeydew, the pathogen produces light pink to cream-colored aerial mycelium, normally at the stem end or the blossom end (Fig. 5). Internally the decay appears as a dry, brown, spongy rot with a white halo (Fig. 2). The postharvest phase in cucumber and squash is normally associated with chilling injury or extended storage. Internally, the lesions remain white and spongy, and aerial mycelium may or may not develop on the surface. On pumpkin, postharvest lesions can vary in appearance depending on the Fusarium species. Dry, pitted lesions are characteristic of F. acuminatum, while F. avenaceum and F. graminearum commonly cause
lesions with rapidly expanding whitish mycelium (Fig. 6).
The lesions may be sunken.
|
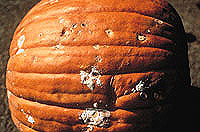
Fig. 6. Lesions caused by (top to bottom) F. acuminatum, F. avenaceum, F. graminearum on pumpkin. Courtesy of W. H. Elmer. (Click image for larger view). |
|
Causal Organisms
F. graminum Corda produces slender, sickle-shaped macroconidia, typically three-septate and occasionally five-septate, having a foot-shaped basal cell. Microconidia and chlamydospores are not produced. Conidiophores may be unbranched or branched monophialides. The sexual stage is not known.
F. graminearum Schwabe produces macroconidia that are thick-walled, straight to moderately sickle-shaped, and unequally curved, with the ventral surface almost straight and the dorsal side arched. No microconidia are present, and chlamydospores are slow to form in culture. Conidiophores are unbranched or branched monophialides. Gibberella zeae (Schwein.) Petch is the perfect stage.
F. acuminatum Ellis & Everh. sensu Gordon produces microconidia only sparsely or not at all and strongly curved macroconidia, whose widest part is one-third the distance from the base to the tip. The basal cell is distinctly foot-shaped. Conidiophores are branched or unbranched monophialides. Chlamydospores may be formed singly, in clumps, or in chains. G. acuminata Wollenweb. is the perfect stage.
F. avenaceum (Fr.:Fr.) Sacc. produces long, slender, thin-walled macroconidia. The apical cell may be bent. Microconidia are scarce, and chlamydospores are present. Conidia are produced on unbranched or branched monophialides. This species resembles F. graminearum but can be differentiated by the larger number of septations in its macroconidia.
F. culmorum (W. G. Sm.) Sacc. produces distinctly septate, stout, thick-walled macroconidia, but no microconidia. The basal cell may range from slightly notched to distinctly foot-shaped. Conidiophores are branched or unbranched monophialides. Chlamydospores may occur singly, in clumps, or in chains. There is no known sexual stage.
F. moniliforme J. Sheld. produces abundant microconidia, which are primarily single-celled and oval to club-shaped. They are formed in long chains and in false heads. Macroconidia are produced but may be rare; they are sickle-shaped to almost straight and have a foot-shaped basal cell. Conidiophores are branched or unbranched monophialides. Dark blue sclerotia may be produced, but chlamydospores are absent. G. fujikuroi (Sawada) Ito is the perfect stage. F. moniliforme can be confused with F. oxysporum, but F. oxysporum does not produce microconidia in chains and does produce chlamydospores.
F. semitectum Berk. & Ravenel rarely produces microconidia, but it can produce two types of macroconidia: sickle-shaped macroconidia, borne in sporodochia, and spindle-shaped to slightly curved macroconidia, borne on aerial mycelium. The basal cell of the spindle-shaped macroconidia is papillate rather than foot-shaped. Conidiophores are unbranched or branched monophialides and polyphialides. Chlamydospores may be present, but there is no known sexual stage. F. semitectum resembles F. equiseti in colony color and morphology, but F. equiseti does not produce spindle-shaped macroconidia or polyphialides.
F. equiseti (Corda) Sacc. sensu Gordon may produce oval to comma-shaped microconidia in aerial mycelium. Macroconidia are strongly septate and sickle-shaped. The basal cell is distinctly foot-shaped. Conidiophores are branched or unbranched monophialides. Chlamydospores with thick, roughened walls are abundant in clumps or chains. On potato-dextrose agar the culture looks similar to F. semitectum, but F. equiseti lacks polyphialides. G. intricans Wollenweb. is the perfect stage.
F. scirpi Lambotte & Fautrey produces ellipsoidal to club-shaped microconidia with zero to three septations. Macroconidia are sickle-shaped, distinctly septate, and thick-walled, resembling those of F. equiseti. The basal cell is distinctly foot-shaped. Conidiophores are branched or unbranched monophialides or are truncate or cross-shaped polyphialides bearing microconidia. Chlamydospores may be produced in clumps or in chains. There is no known perfect stage. F. equiseti and F. semitectum may be similar in some respects to F. scirpi. However, only F. scirpi produces microconidia borne on polyphialides.
F. solani (Mart.) Appel & Wollenweb. emend. W. C. Snyder & H. N. Hans. produces oval to kidney-shaped and generally single-celled microconidia. Macroconidia are stout and generally cylindrical. The apical cell is blunt and rounded, and the basal cell may be distinctly foot-shaped, notched, or rounded. Conidiophores are branched or unbranched monophialides and are longer than those of F. oxysporum. Chlamydospores are formed singly and in pairs.
F. oxysporum Schlechtend.:Fr. f. sp. melonis (Leach & Currence) W. C. Snyder & H. N. Hans. is described in another section of the Compendium of Cucurbit Diseases (see Fusarium Wilt of Melon).
Disease Cycle
Fruits of all cucurbits are susceptible to one or more species of Fusarium. The fungus may penetrate directly under moist or wet conditions. Wounds facilitate fungal entry. Most infections of fruit occur in the region that is in contact with the soil. Although uncommon in watermelon fruit, Fusarium spp. can infect at the stem end and, less frequently, at the blossom end and belly. Fusarium rot is a fairly common fruit rot of pumpkin and squash, as both a preharvest and a postharvest decay (see Fusarium Crown and Foot Rot of Squash). In cucumber, the postharvest disease tends to be more severe following chill injury. Fusarium rot is common in honeydew melons, occurring most frequently on the stem end, although the exact mode of infection is not well understood. Natural infection of melon fruit by Fusarium spp. appears to be related to net development. Once callus tissue develops in the netted area (about 25 days), further infection is probably greatly reduced. Colonization of the tissue is slow until fruit maturity. Large numbers of conidia are produced on field-culled and unharvested melons. Little information is available on the epidemiology of Fusarium rot of cucurbits. Many of the fruit-rotting Fusarium spp. are reported to be seedborne.
Control
Most infections of cucurbits by Fusarium spp. occur in the field (preharvest) and, to a lesser extent, during harvesting and handling. Preharvest fungicide application has been somewhat ineffective, because of difficulty in obtaining sufficient coverage of the fruit. Postharvest control of Fusarium rot of melon has also been erratic.
Fungicides in combination with hot-water treatment have generally been successful in controlling Fusarium fruit rot. The duration of immersion (1 min) and the temperature (57°C) are critical for adequate control. Immersion for a period of less than 1 min is ineffective. Numerous hypotheses have evolved about the benefits of combining a hot-water treatment with a fungicide. Results of studies on the combined effects are inconclusive. Both hot-water treatment and fungicide are beneficial individually. More recently, research has demonstrated that the mechanism for improved postharvest disease control in melons from the hot-water and fungicide combination is due to increased fungicide penetration.
Avoidance of wounding during harvest and packing, proper storage and transit temperatures, and prompt handling of melons upon arrival at the market provide some protection against postharvest decay.
Selected References
Carter, W. W. 1979. Corky dry rot of cantaloup caused by Fusarium roseum 'Semitectum.' Plant Dis. Rep. 63:1080-1084.
Carter, W. W. 1981. Reevaluation of heated water dip as a postharvest treatment for controlling surface and decay fungi of muskmelon fruits. HortScience 16:334-335.
Nelson, P. E., Toussoun, T. A., and Marasas, W. F. O. 1983. Fusarium Species: An Illustrated Manual for Identification. Pennsylvania State University Press, University Park.
Stewart, J. K., and Wells, J. M. 1970. Heat and fungicide treatments to control decay of cantaloupes. J. Am. Soc. Hortic. Sci. 95:226-229.
Waraitch, K. S., and Nandpuri, K. S. 1975. Fusarium fruit rot of muskmelon (Cucumis melo L.). J. Res. Punjab Agric. Univ. 12:131-134.
Wiant, J. S. 1937. Investigations of the market diseases of cantaloups and honey dew and honey ball melons. U.S. Dep. Agric. Tech. Bull. 573.
RETURN TO APSnet FEATURE STORY
