Peer-Reviewed by Plant Health Progress
Accepted for publication 10 May 2001. © 2001 Plant Health Progress.
Wade H. Elmer, Connecticut Agricultural Experiment Station, New Haven, CT 06504
Corresponding author: Wade H. Elmer. wade.elmer@po.state.ct.us
Elmer, W. H. 2001. The economically important diseases of asparagus in the U.S. Online. Plant Health Progress doi:10.1094/PHP-2001-0521-01-RV.

Fig. 1. Asparagus spears (courtesy of R. Mullen) (click image for larger view). |
|
In the central and eastern U.S., the months of April and May bring more than showers and flowers. They mark the beginning of asparagus (Asparagus officinalis L.) season (Fig. 1). Asparagus (Greek for “shoot”) has long been a delicacy coveted by many cultures. It is highly nutritious, flavorful, and has medicinal properties (69). It acts as a strong diuretic and benefits people with heart problems. Many claim it is an aphrodisiac.
Asparagus is usually established with 1-year-old plants commonly referred to as crowns that include buds and fleshy storage roots (Fig. 2). Marketable spears are first harvested after crowns have been established in the field for 2-3 years. Spears are cut and trimmed to 18-22 cm in length. Cutting occurs every 24-48 hr. (Fig. 3), and harvest periods can last up to 2 months. Americans are now consuming more green asparagus than ever before. Despite the high demand, asparagus acreage in the U.S. has declined by 20% from 37,840 ha in 1990 to 30,356 ha in 1999 (1). Duty-free imports from South America have seriously threatened the U.S. industry. Keeping U.S. growers competitive will require government, extension, and research efforts. This paper presents a review of the major diseases of asparagus that routinely cause losses in the U.S. and reviews their management strategies.
|

|
|

|
|
Fig. 2. Asparagus crowns being planted in trenches (courtesy of T. Reid and M. Hausbeck) (click image for larger view). |
|
Fig. 3. Asparagus harvest (courtesy of T. Reid and M. Hausbeck) (click image for larger view). |
Asparagus History
Asparagus probably evolved in the eastern regions of the Middle East (3). Some people credit Alexander the Great with discovering asparagus during his exploits and introducing it to Greece around 300 BC. Romans rapidly adopted the crop. In 160 BC, Porcius Cato the Elder published his De Agri Cultura, which described growing methods for asparagus. Pliny the Elder’s book, Natural History (57 AD), devoted several discussions to the medicinal properties of asparagus. Surprisingly, interest in asparagus declined in the Middle Ages until the 1600s. Revived interest, due in part to Louis IV’s fondness for the plant, led to increased production in France (69).
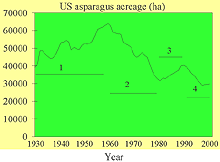
Fig. 4. U.S. acreage planted to asparagus from 1930-2000. Intervals represent: (1) period of increased acreage due to advances in canning and transportation; (2) period of decline due to Fusarium crown and root rot; (3) period of increased acreage due to new cultivars and increased demand; and (4) period of decline presumably due to competition from imports (click image for larger view). |
|
Advances in processing and transportation in the 1930s led to annual increases in U.S. (Fig 4). Acreage was concentrated in California, Washington, and Michigan. During 1960 to 1980, a sharp decline in acreage occurred. The loss had devastating effects on growers in the eastern U.S. However, increased demand and new cultivars revived the industry in the 1980s. Dr. Howard Ellison of Rutgers University selfed hermaphrodites (male plants with functional female flower parts) to obtain several males that were homozygous for maleness. Tissue-cultured super males (homozygous males) are used to produce an all-male hybrid when crossed with a female parent plant. In 1985, the all-male hybrid, ‘Jersey Giant,’ was released (16), and currently all-male hybrids are being produced around the world. Open-pollinated hybrids, such as UC 157 are still very popular and better adapted to Mediterranean and desert conditions.
Although demand is still high in the U.S., acreage has shown an alarming decline over the last decade (Fig. 4). Politics may have played a role. Government programs, such as the Agency for International Development that fund large asparagus projects abroad and free trade agreements such as NAFTA and the Andean Trade Preference Act, have eliminated tariffs on asparagus imports leading to a year-round supply of asparagus in U.S. markets. Asparagus imports have risen from 23,978 metric tons in 1992 to 33,901 metric tons in 1997 (64). The ability of exporting countries to cheaply produce the crop year-round has made serious inroads into U.S. production. For example, in the last decade frozen asparagus imports from South America have essentially displaced the frozen asparagus industry in the U.S. Other U.S. programs, such as the Market Access Program, have promoted U.S. asparagus, but growers are faced with the realization that they must reduce costs to remain competitive. Although labor remains the major cost factor for U.S. growers, pest management still requires costly inputs. This article will review the economically limiting diseases of asparagus in the U.S. and explore current and future options for improved management.
Asparagus Rust

Fig. 5. Drawing of Puccinia asparagi spore types (click image for larger view and more information). |
|
Asparagus rust, caused by Puccinia asparagi DC. in Lam. & DC., was originally described in France in 1805, and appeared in New Jersey in 1896 (39). Within a few years, rust had spread to every region where asparagus was grown (54,65). P. asparagi is an autoecious (single host), pathogen which produces four spore types (basidiospores, aeciospores, urediniospores, and teliospores) in succession (Fig. 5). Early spring infections of young spears occur with basidiospores. These infections, in turn, produce oval, light-green lesions about 6 x 19 mm in size. One to 2-weeks later, cream colored aecia are produced (Fig. 6). The wind-blown aeciospores initiate early rust epidemics and then give rise to the brownish-red uredinia stage (Fig. 7). The uredinial cycle repeats every 10 to 14 days during July and August (47) and causes the majority of fern damage. In early fall, telia replace uredinia and serve as the overwintering stage. Because first year fields are not harvested, the aecia stage on the spear is not removed and leads to greater severity than in fields being cut. The difference in rust severity between a 2-year-old uncut field and a 3-year-old harvested field can be striking (Fig. 8).
|

Fig. 6. Aecial lesion of Puccinia asparagi on the stem of asparagus (courtesy of D. Johnson) (click image for larger view).
|
|

Fig. 7. Uredinia of Puccinia asparagi on the stem of asparagus (courtesy of D. Johnson) (click image for larger view). |
|

Fig. 8. Field of ‘Delmonte 361’ asparagus. Field on the left is 2 years old and was not harvested and aecial lesions developed; field on the right is 3 years old and was harvested preventing the aecial stage from developing (courtesy of D. Johnson) (click image for larger view).
|
|
Management of rust in the U.S. has been achieved using an integrated approach of resistant cultivars, fungicides, and sanitation practices. Breeders have since made great improvements in incorporating stable quantitatively inherited resistance into commercial lines (4,42,45,51,52). However, the current level of resistance is insufficient to completely control severe outbreaks (46). Cultivar improvements are needed. Because damage thresholds have been determined (21,49), fungicide applications could be timed to suppress outbreaks during conducive periods (49). The current dilemma in rust management is the loss of registered products and/or the processors refusal to accept asparagus treated with EBDC fungicides. Special use permits under Section 18 have allowed new unregistered products to be used, but these costly inputs could be reduced with improved genetic resistance to rust.
Fusarium crown and root rot
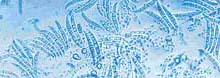
The disease was first noted in 1908 (70). Over the years the disease has been called dwarf asparagus (9), wilt and root rot (8), seedling blight (38), crown rot complex (27), and Fusarium stem and crown rot (53). The disease affects seedlings (Fig. 9) and mature plants (Fig. 10 and 11). The first Fusarium pathogen found associated with the disease was Fusarium oxysporum (Schlecht) f. sp. asparagi (S. I. Cohen) in 1941 (8) (Fig. 12). Gibberella fujikuroi (Sawada) Ito in Ito & K. Kimera [(Mating Group “D”), anamorph F. proliferatum (Synamorph F. moniliforme)] was found to be a pathogen as well in 1979 (53) (Fig. 13). Pathogens are seedborne (12,43) and carried on transplants. The disease became economically limiting in the 1950’s (38,39). U.S. acreage declined annually from 1960 to 1980 (Fig. 4). Many growers in the eastern U.S. were forced to abandoned asparagus as a crop.

Fig. 9. Seedling blight stage of Fusarium crown and root rot of asparagus (courtesy of T. Reid and M. Hausbeck) (click image for larger view). |

Fig. 10. Mid season symptoms of Fusarium crown and root rot on mature plants (courtesy of J. LaMondia) (click image for larger view). |
|
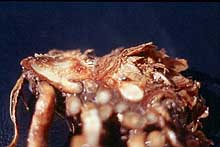
Fig. 11. Symptoms of Fusarium crown and root rot of asparagus showing vascular discoloration in the crown (click image for larger view). |
 |
|
|
 |
|
Fig. 12. Macroconidia, microconidia, and chlamydospores of Fusarium oxysporum f. sp. asparagi (courtesy of T. Reid and M. Hausbeck) (click image for larger view).
|
|
|
Fig. 13. Chains of microconidia on a conidiophore and macroconidia of Fusarium proliferatum (courtesy of T. Reid and M. Hausbeck) (click image for larger view).
|
F. oxysporum f. sp. asparagi appears to be present in most asparagus field soils (13,40). The pathogen represents a group that contains many genetically distinct members (5,24,57). Pathogenicity on asparagus may be an unspecialized trait that appears frequently in F. oxysporum. On the other hand, F. proliferatum appears to belong to one mating group (15,19,25). Because perithecia are not found in nature, it is assumed that the asexual stage of F. proliferatum is responsible for dispersal. Using heterokaryon tests to follow vegetatively compatible groups, one group (US5) has been found to predominate in the U.S. and Australia (17,26). Because seed transmission is the likely method of dissemination, simple seed disinfestation alone could slow the spread of Fusarium pathogens to new areas (12,23,43).
Management of Fusarium crown and root rot has been difficult. Pathogens are ubiquitous (13,25,57). Preventative measures, such as choosing sandy, well-drained soils and selecting the best cultivars for the area, are very important, but growers need strategies in place when established plantings show signs of decline. The resistance in the all-male hybrids has helped to reduce the financial losses due to this disease, but improvements are still needed that will ensure plantings' greater longevity. Fungicide applications have traditionally been ineffective or impractical (34,55,59). Nigh (63) stated that any stress factor, such as drought or weeds, will increase the incidence and severity of Fusarium crown and root rot. As a result, management programs that control insects and weeds will reduce damage from Fusarium crown and root rot (14). Although asparagus is drought-tolerant, small deficits in soil moisture can result in large reductions in growth (74) and increases in infection (22). Cutting pressure can also exert stress on a production field; consequently, extending the harvest season weakens the plant’s ability to regenerate the stems and ferns that produce the carbohydrates for next year’s yield (68) and should be avoided.
Limited success has been made by broadcasting NaCl onto older, declining fields (18,20). The practice of salting asparagus beds was probably used from before 1860 to around the 1940’s to control weeds and boost yields (6,7,67,72,73). It appears to be a practice unique to the U.S. and was discontinued after herbicides were developed in the 1940s. About this time the number of reports of Fusarium crown and root rot in asparagus began to increase (8,37,38). Research has found that rates between 560-1120 kg NaCl/ha will boost vigor, slow the rate of decline (18), and may allow growers to recoup some profits for a negligible cost. The mechanism of NaCl on Fusarium crown and root rot is unclear. It does not affect soil pathogens (18), and may improve host defense (20). The treatment offers little benefit to healthy asparagus and may not have value as a preventative strategy. Information about timing and rates are still needed, but concern about environmental issues and salt damage has delayed its acceptance as a management strategy.
Purple Spot
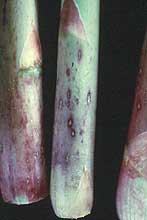
Purple spot, which is caused by Pleospora herbarium (anamorph Stemphylium vesicarium), was first reported in the U.S. in 1981 (56). The disease has since been reported in all major asparagus-growing regions in the U.S. (33,50). In addition, the disease has recently become more damaging (41). It was first reported as small (1-2 mm), elliptical, slightly sunken, purplish spots that blemished the spears and lowered its marketability (Fig. 14). Today, the major damage from purple spot is on the fern growth (Fig. 15). Damage to the fern results in defoliation of the needle-like cladophylls that reduces the flow of carbohydrates to the roots and lowers next year’s yield (41). It is not known if the resistance to purple spot has been inadvertently reduced through breeding or if the inoculum levels have reached threshold densities. No-till asparagus culture may have contributed to the increase in purple spot by allowing colonized residues to remain in the field (41,66).
 |
|
 |
|
Fig. 14. Purple spot lesions caused by Pleospora herbarium on asparagus spears (courtesy of D. Johnson) (click image for larger view). |
|
Fig. 15. Asparagus fern showing purple spot lesions caused by Pleospora herbarium (click image for larger view).
|
Spear infections are favored by wet, cool weather and by wounding from wind-driven sand (50). During a harvest period in Connecticut, spears were free of purple spot until two days of wet and cold weather occurred, and then incidence increased to 100 % (Fig. 16); when weather conditions improved, new spears remained disease free. Current management of purple spot has focused on sanitation and fungicides. Removing the previous year's fern growth can reduce severity of purple spot on spears during harvest (48). Chopping and incorporating the stalks in the fall can prevent ascospores and conidia from becoming airborne, but the practice does not result in decomposition of the pseudothecia before the spring harvest. However, this practice must be weighed against the potential damage caused to the roots and crown that can favor Fusarium infections (66). Cover crop mulches and wind barriers that reduce blowing sand need investigation. Additionally, the fungicide chlorothalonil has been effective when applied to the fern using the FAST disease forecasting system (58). Benefits in savings between $1,000-$2,000 per ha have been documented in Michigan (60). A further reduction in inputs could be achieved by increasing cultivar resistance to purple spot.
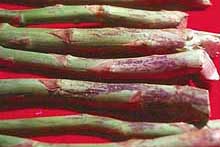
Fig. 16. Purple spot lesions caused by Pleospora herbarium on asparagus spears harvested during cool wet weather (click image for larger view). |
Asparagus Viruses
The first study demonstrating the effect of viruses on asparagus decline was done in New Jersey (75). A decline in productivity was correlated with the presence of viral agents in young plantings. Three viral agents are known to infect asparagus in the U.S. All three viruses, asparagus virus I (AV-I), asparagus virus II (AV-II), and tobacco streak virus (TSV), appear to be widely distributed in commercial asparagus plantings (29,32,62). However, not all are equal in the damage they cause. None of the three viruses produces distinctive symptoms on asparagus. The damage is exhibited as reduced vigor and increased susceptibility to other diseases (30).
Under field conditions, AV-I infects only asparagus and is transmitted by a wide variety of aphid species in the stylet-borne manner, but efforts to suppress aphids have little effect on the spread of AV-I (61). The virus alone has little effect on plant growth, yield of spears, or longevity of the plant. It is an economic factor because it interacts with AV-II to produce more damage than with AV-II alone (21). Because AV-I is not seedborne, growers can be assured their new plantings are initially free of AV-I. Nevertheless, in Washington, a study found that all plants tend to get AV-I over time (21). AV-II occurs more frequently than AV-I and is more damaging. It is readily transmitted through seed from infected parents and from seed produced on healthy plants that were fertilized by AV-II-contaminated pollen (28,71). Due to routine indexing, only trace amounts of the AV-II are being found. AV-II was presumed to spread very slowly in the field (29). However, studies in New Zealand provided strong evidence that spread of AV-II was occurring during harvest on cutting knives (44). This observation warrants close attention to the possible spread of AV-II in production fields in the U.S. TSV, the second most common ilarvirus in asparagus, is not seedborne, but can spread presumably through thrips-mediated pollen transmission (61). Its contribution to yield loss is minor unless there is prior infection by AV-II. Because AV-II is the major threat, whether by itself or combined with AV-I and TSV, monitoring its presence and spread should become a routine practice. Rapid viral assays may help to index fields at risk and alert growers to use sanitation procedures during harvest.
Phytophthora Spear Rot

Fig. 17. Field symptoms of Phytophthora rot of asparagus (courtesy of R. Mullen) (click image for larger view). |
|
Phytophthora spear rot was first described in California in 1938 (2) but was not thoroughly studied again until the 1980’s when 30-54% reductions in yield were documented (31) (Fig. 17). The fungus most associated with the disease is Phytophthora megasperma Drechs., although other Phytophthora species have been found (2). Symptoms include soft, water-soaked lesions on shoots at, slightly above, or below the soil level (Fig. 18). As the lesions expand, they turn light brown, collapse, and shrivel. This flattens the affected side of the spear, and the spear bends (Fig. 19). The internal tissues of infected crowns may be a yellow-brown color (Fig. 20).

Fig. 18. Healthy asparagus spear and spear infected with Phytophthora rot (courtesy of R. Mullen) (click image for larger view). |
|

Fig. 19. Bent spear symptoms of Phytophthora rot on asparagus in field (courtesy of R. Mullen) (click image for larger view). |

Fig. 20. Phytophthora crown rot of asparagus revealing the yellow-orange coloration to the roots (courtesy of R. Mullen) (click image for larger view).
|
The damage caused by Phytophthora rot can vary from year to year and site to site and depends on rainfall and soil drainage. The disease is more prevalent in warmer regions of the U.S. and has not been a problem in northern areas. Fungicide applications are helpful in establishing beds when wet weather occurs (35). Products such as metalaxyl (currently only available as the active component, mefenoxam) are effective (35,36), but concerns about pathogen resistance warrants the pursuit of other products and other strategies. Trials to identify resistance to Phytophthora rot have commenced in California.
Cercospora Blight
Another foliar disease that contributes to losses in asparagus in warm, humid environments is Cercospora blight, caused by Cercospora asparagi Sacc. It has been severe in eastern Oklahoma (10) and North Carolina (11), but does not cause appreciable damage in cooler or dryer climates.
Lesions are small, oval, grayish-tan in color, with purple borders, and can be confused with those caused by P. herbarium (Fig. 21). Blighted ferns turn yellow to brown and eventually die prematurely (11) (Fig. 22). Since there are no known sources of resistance to Cercospora blight, management must include sanitation and fungicide applications. Fall removal or burial of the year's fern residue can delay the onset of disease development.
 |
|
 |
|
Fig. 21. Symptoms of Cercospora blight on a stem of asparagus (courtesy of D. Sanders and C. Averre) (click image for larger view). |
|
Fig. 22. Defoliation of the lower canopy of asparagus caused by Cercospora blight (courtesy of D. Sanders and C. Averre) (click image for larger view).
|
Fungicides are also an important component of an integrated approach and can increase spear production the following year (10). The inclusion of disease forecasting models may reduce the number of applications and save cost. Research on cultivar resistance is in its infancy.
Summary
The amount of asparagus consumed in the U.S. will undoubtedly continue to increase as availability of the crop increases. However, the full effect of imports on the U.S. industry may not be seen for some time. Industry-sponsored groups, such as Asparagus USA, have made progress in promoting U.S. asparagus abroad and educating government agencies of the economic situation in the U.S. The imported shipments from South America have already diminished the frozen asparagus industry and have begun to make inroads into canned and fresh markets as well. It is also alarming that China committed approximately 54,000 ha to asparagus during the 1992-1997 period (63). Given the perishability of the crop, local fresh asparagus markets will still have some quality advantages provided consumers will pay for that difference.
The development of genetically resistant material has always been a major area of asparagus research. The success of the all-male hybrids has been a tremendous asset to the industry, but increased disease resistance is still needed. As genetic modifications in plants become more advanced, the future should offer many solutions to the problems in asparagus. A concerted effort between breeders and plant health specialists must be maintained and supported to improve genetic resistance to AV-II, purple spot, Phytophthora spear rot, and Cercospora blight. Given the perennial nature of the crop, evaluations require long term-support from private and public sectors.
Additional Resources
Asparagus Research NewsletterCalifornia Asparagus Commission
Michigan Asparagus Advisory Board
Stockton Asparagus Festival
Washington Asparagus Commision
International Society for Horticultural Science
Literature Cited
1. Anonymous. 1960-2000. Agricultural Statistics. USDA. U.S. Government Printing Office, Washington, D.C.
2. Ark, P. A., and Barrett, J. T. 1938. Phytophthora rot of asparagus rot in California. Phytopathology 28:754-756.
3. Bailey, L. H., and Bailey, E. Z. 1976. Hortus Third: A Concise Dictionary of Plants Cultivated in the United States and Canada. MacMillan Publishing Co., New York.
4. Blanchette, B. L., Groth, J. V., and Waters, L., Jr. 1982. Evaluation of asparagus for resistance to Puccinia asparagi. Plant Dis. 66:904-906.
5. Blok, W. J., and Bollen, G. J. 1997. Host specificity and vegetative compatibility of Dutch isolates of Fusarium oxysporum f.sp. asparagi. Can. J. Bot. 75:383-393.
6. Brill, A. B. 1872. Farm Gardening and Seed Growing. Orange Judd Co., New York.
7. Burr, F. 1865. Garden Vegetables and How to Cultivate Them. S. W. Tilton, Boston.
8. Cohen, S. I., and Heald, F. D. 1941. A wilt and root rot of asparagus caused by Fusarium oxysporum Schlecht. Plant Dis. Reptr. 25:503-509.
9. Cook, M. T. 1923. Dwarf asparagus. Phytopathology 13:284.
10. Conway, K. E., Motes, J. E., and Foor, C. J. 1990. Comparison of chemical and cultural controls for Cercospora blight on asparagus and correlations between disease levels and yield. Phytopathology 80:1103-1108.
11. Cooperman, C. J., Jenkins, S. F., and Averre, C. W. 1986. Overwintering and aerobiology of Cercospora asparagi in North Carolina. Plant Dis. 70:392-394.
12. Damicone, J. P., Cooley, D. R., and Manning, W. J. 1981. Benomyl in acetone eradicates Fusarium moniliforme and F. oxysporum from asparagus seed. Plant Dis. 65:892-893.
13. Damicone, J. P., and Manning, W. J. 1985. Frequency and pathogenicity of Fusarium spp. isolated from first-year asparagus grown from transplants. Plant Dis. 69:413-416.
14. Damicone, J. P., Manning, W. J., and Ferro, D. N. 1987. Influence of management practices on severity of stem and crown rot, incidence of asparagus miner, and yield of asparagus grown from transplants. Plant Dis. 71:81-84.
15. Doan, M. C., and Carris, L. M. 1998. Characterization of Fusarium populations in asparagus fields in the Pacific Northwest. Acta Horticulturae 479:219-226.
16. Ellison, J. H., and Kinelski, J. J. 1985. 'Jersey Giant': an all male asparagus hybrid. HortSci. 20:1141.
17. Elmer, W. H. 1991. Vegetative compatibility groups of Fusarium proliferatum from asparagus and comparisons of virulence, growth rates, and colonization of asparagus residues among groups. Phytopathology 81:852-857.
18. Elmer, W. H. 1992. Suppression of Fusarium crown and root rot of asparagus with sodium chloride. Phytopathology 82:97-104.
19. Elmer, W. H. 1995. A single mating population of Gibberella fujikuroi (Fusarium proliferatum) predominates in asparagus fields in Connecticut, Massachusetts, and Michigan. Mycologia 87:68-71.
20. Elmer, W. H. 1995. Association between Mn-reducing root bacteria and NaCl applications in suppression of Fusarium crown and root rot of asparagus. Phytopathology 85:1461-1467.
21. Elmer, W. H., Johnson, D. A., and Mink, G. I. 1996. Epidemiology and management of the diseases causal to asparagus decline. Plant Dis. 80:117-125.
22. Elmer, W. H., and LaMondia, J. A. 1998. Studies on the suppression of Fusarium crown and root rot with NaCl. Acta Horticulturae 479:211-215.
23. Elmer, W. H., and Stephens, C. T. 1988. Comparison of techniques for eliminating contaminants from asparagus seeds. HortScience 23:1031-1032.
24. Elmer, W. H., and Stephens, C. T. 1989. Classification of Fusarium oxysporum f. sp. asparagi into vegetatively compatible groups. Phytopathology 79:88-93.
25. Elmer, W. H., Summerell, B. A., Burgess, L.W., Backhouse, D., and Abubaker, A.A. 1997. Fusarium species associated with asparagus crowns and soil in Australia and New Zealand. Australasian Pl. Path. 26:255-261.
26. Elmer, W. H., Summerell, B. A., Burgess, L. W., and Nigh, E. L. 1999. Vegetative compatibility groups of Fusarium proliferatum from asparagus in Australia Mycologia 91:650-654.
27. Endo, R. M., and Burkholder, E. C. 1971. The association of Fusarium moniliforme with the crown rot complex of asparagus Phytopathology (Abstr.) 61:891.
28. Evans, T. A., and Stephens, C. T. 1988. Association of asparagus virus II with pollen from infected plants. Plant Dis. 72:195-198.
29. Evans, T. A., DeVries, R. M., Wacker, T. L., and Stephens, C. T. 1989. Epidemiology of asparagus viruses in Michigan asparagus. Acta Horticulturae 271:285-290.
30. Evans, T. A., and Stephens, C. T. 1989. Increased susceptibility to Fusarium crown and root rot in virus-infected asparagus. Phytopathology 79:253-258.
31. Falloon, P. G., Falloon, L. M., Benson, B. L., and Grogan, R. G. 1986. Effect of Phytophthora megaspermae var sojae on yield of Asparagus officinalis. Plant Dis. 70:15-19.
32. Falloon, P. G., Falloon, L. M., and Grogan, R. G. 1986. Survey of California asparagus for asparagus virus I, asparagus virus II, and tobacco streak virus. Plant Dis. 70:103-105.
33. Falloon, P. G., Falloon, L. M., and Grogan, R. G. 1987. Etiology and epidemiology of Stemphylium leaf spot and purple spot of asparagus in California. Phytopathology 77:407-413.
34. Falloon, P. G., Fraser, H., and Nikologg, A. S. 1989. Control of Fusarium rot with Thiabendazole. Final Report. Crop Research Division, DSIR, Christchurch, NZ.11p.
35. Falloon, P. G., Greathead, A. S., Mullen, R. J., Benson, B. L., and Grogan, R. G. 1991. Individual and combined effects of flooding, Phytophthora rot, and metalaxyl on asparagus establishment. Plant Dis. 75:514-518.
36. Falloon, P. G., Mullen R. J., Benson, B. L., and Grogan, R. G. 1985. Control of Phytophthora rot with metalaxyl in established asparagus. Plant Dis. 69:921-923.
37. Graham, K. M. 1955. Seedling blight, a fusarial disease of asparagus. Can. J. Bot. 33:374-400.
38. Grogan, R. G., and Kimble, K. A. 1959. The association of Fusarium wilt with the asparagus decline and replant problem in California. Phytopathology 49:122-125.
39. Halstead, B.D. 1898. The asparagus rust: Its treatment and natural enemies. NJ Agr, Exp. Sta. Bull. 129 20p.
40. Hartung, A. C., Stephens, C. T., and Elmer, W. H. 1990. Survey of Fusarium populations in Michigan's asparagus fields. Acta Horticulturae 271:395-401.
41. Hausbeck, M. K., Hartwell, J., and Byrne, J. M. 1997. Epidemiology of Stemphylium leaf spot in no-till asparagus. Acta Horticulturae 479:205-210.
42. Hepler, P. R., Thompson, A. E., and McCallum, J. P. 1957. Inheritance of resistance to asparagus rust. Illinois Agric. Exp. Stn. Bull. 607. 47 p.
43. Inglis, D. A. 1980. Contamination of asparagus seed by Fusarium oxysporum f. sp. asparagi and Fusarium moniliforme. Plant Dis. 64:74-76.
44. Jasper, M. V., and Falloon, P. G. 1999. Asparagus virus 2: A contributing factor in asparagus decline. Acta Horticulturae 479:263-270.
45. Johnson, D. A. 1986. Two components of slow-rusting in asparagus infected with Puccinia asparagi. Phytopathology 76:208-211.
46. Johnson, D. A. 1989. Variation for rust resistance within asparagus cultivars. Plant Dis. 73:309-312.
47. Johnson, D. A. 1990. Development of rust on asparagus cultivars after inoculation with basidiospores, aeciospores, and urediniospores of Puccinia asparagi. Phytopathology 80:321-325.
48. Johnson, D. A. 1990. Effect of crop debris management on severity of Stemphylium purple spot of asparagus. Plant Dis. 74:413-415.
49. Johnson, D. A. 1992. Asparagus Rust. Coop. Ext. Wash. State Univ. EB 1656. 4 p.
50. Johnson, D. A., and Lunden, J. D. 1986. Effects of wounding and wetting duration on infection of asparagus by Stemphyllium vesicarium. Plant Dis. 70:419-420.
51. Johnson, D. A., and Lunden, J. D. 1992. Effect of rust on yield of susceptible and resistant asparagus cultivars. Plant Dis. 76:84-86.
52. Johnson, D. A., and Peaden, R. N. 1993. Rust resistance in asparagus F1 hybrid populations. Plant Dis. 77:1144-1148.
53. Johnston, S. A., Springer, J. K., and Lewis, G. D. 1979. Fusarium moniliforme as a cause of stem and crown rot of asparagus and its association with asparagus decline. Phytopathology 69:778-780.
54. Kahn, R. P., Anderson, H. W., Hepler, P. R., and Linn, M. B. 1952. An investigation of asparagus rust in Illinois, its causal agent and its control. Illinois Agric. Exp. Stn. Bull. 559. 59 p.
55. Lacy, M. L. 1979. Effects of chemicals on stand establishment and yields of asparagus. Plant Dis. Rept. 63:612-616.
56. Lacy, M. L. 1982. Purple spot: a new disease of young asparagus spears. caused by Stemphylium vesicarium. Plant Dis. 66:1198-1200.
57. LaMondia, J. A., and Elmer, W. H. 1989. Pathogenicity and vegetative compatibility among isolates of Fusarium oxysporum and F. moniliforme colonizing asparagus tissues. Can. J. Bot. 67:2420-2424.
58. Madden, L., Pennypacker, S. P., and McNab, A. A. 1978. FAST: a forecast system for Alternaria solani on tomato. Phytopathology 68:1354-1358.
59. Manning, W. J., and Vardaro, P. M. 1977. Soil fumigation and preplant fungicide crown soaks: Effects on plant growth and Fusarium incidence in newly planted asparagus. Plant Dis. Rept. 61:355-357.
60. Meyer M. P., Hausbeck, M. K., and Podolsky, R. 2000. Optimal fungicide management of purple spot of asparagus and impact on yield. Plant Dis. 84:525-530.
61. Mink, G. I. 1992. Ilarvirus vectors. Adv. Dis. Vec. Res. 9:267-281.
62. Mink, G. I. and Uyeda, I. 1977. Three mechanically transmitted viruses isolated from asparagus in Washington. Plant Dis. Rept. 61:398-401.
63. Nigh, E. L.1990. Stress factors influencing Fusarium infection in asparagus. Acta Horticulturae 271:315-322.
64. Nigh, E. L. 1999. The explosion of new asparagus production worldwide and what it means. Acta Horticulturae 479:11-15.
65. Norton, J. B. 1913. Methods used for breeding asparagus for rust resistance. U.S. Bur. Plant Ind. Bull. 263.
66. Putnam, A. R., and Lacy, M. L. 1977. Asparagus management with no-tillage. MI State Univ. Agric. Exp. Sta. Res. Rep. 339.
67. Ruldolph, W. 1927. Influence of salt upon the growth rate of asparagus. Bot. Gaz. 83:94-98.
68. Shelton, D. R., and Lacy, M. L. 1980. Effect of harvest duration and yield depletion of storage carbohydrate in asparagus roots. J. Am. Soc. Hort. Sci. 105:332-335.
69. Schofield, M. 1946. Asparagus, past and present. Food Manufacture 21:443-445.
70. Stone, G. E., and Chapman, G. H. 1908. Report of the botanist. MA Agr. Exp. Sta. 20:127.
71. Uyeda, I., and Mink, G. I. 1981. Properties of asparagus virus II, a new member of the ilarvirus group. Phytopathology 71:1264-1269.
72. Walker, E. 1905. Asparagus and salt. AK Agric. Exp. Stn. Bull. 86:31-36.
73. White, N. N. 1868. Gardening for the South. Orange Judd. Co., New York.
74. Wilcox-Lee, D. 1987. Soil matrix potential, plant water relations and growth in asparagus. HortSci 22:22-24.
75. Yang, H. 1979. Early effects of viruses on the growth and productivity of asparagus plants. HortSci. 14:734-735.
