Updated 2016.
OBJECTIVES
Learning Objectives--After this exercise, students should be able to:
-
Collect plants and isolate specific members (bacteria) from the root microbiome
-
Perform qualitative plate assays using a reporter strain to identify members of the root microbiome that communicate positively and negatively
-
Handle various microbiology laboratory equipment, including pipettors, microfuges, plate spreaders and shakers
-
Handle sterile laboratory medium aseptically
-
Write a concise laboratory report that includes an introduction and overall concept, the methodology, the results, and a discussion
-
Calculate the frequency of cross-communicators and hypothesize why different plant hosts support different numbers of cross-communicators
-
Think critically about experimental and biological parameters that may influence the experimental results
INTRODUCTION
Throughout most of the modern era of microbiology and plant pathology, scientists have studied plant-associated bacteria after isolating them as pure cultures. This reductionist approach led to incredible breakthroughs in our understanding many basic and critical biological functions applicable to other higher order organisms. An unintended result of this approach was the belief that bacteria act as single cells, each cell sensing and responding to its environment independently. Although we have long known microorganisms do not exist isolated in nature, but exist in complex communities now known as the microbiome, many scientists felt the study of the microbiome was so complex that the results obtained would be undecipherable. However, studies that do not consider the influence of the microbiome by default must be incomplete. Increasing evidence over the past 30 years is showing that although bacteria have the ability to react independently, in nature they do not act as single cells but have the ability to act as a population analogous to a multicellular organism (Shapiro 1998; West et al. 2007; West et al. 2006). This ‘multicellular organism’ can consist of members of the same bacterium or as mixed communities of bacteria and bacteria and other organisms.
In vitro studies of bacteria demonstrated that they utilize a diverse range of signals to detect and communicate environmental parameters. These include their nutritional status, population density, the presence of other members of the microbiome (Gray 1997; Schell 2000; Wirth 2000). These signals modulate the expression of a number of bacterial behaviors, including multicellular differentiation, fruiting body development, and sporulation, as well as processes such as bioluminescence, plasmid conjugal transfer, and secondary metabolism (Table 1).
Table 1. Examples of diffusible signals utilized by bacteria.
| Signal | Mediates |
| Butyrylactones | antibiotic synthesis in
Streptomyces spp. |
Amino acids | swarming in
Protease spp. |
Peptides | - competence in
Bacillus spp.
- fruiting body formation in
Myxococcus spp.
- conjugal plasmid transfer in
Enterococcus spp.
|
|
Dialkylresorcinols, cyclohexanones |
cell clumping in
Photorhabdus spp. |
|
N-acyl-homoserine lactones (HSLs, AHLs) |
pathogenicity gene expression, secondary
metabolite biosynthesis, etc. in Pseudomonads |
Many excellent reviews have been written on the various types of known signaling molecules produced by bacteria. For this exercise, we will focus on one well-characterized class of diffusible signal molecules known as
N-acyl-homoserine lactone molecules (AHLs)
(Dunlap 1997; Fuqua et al. 1996; Pierson et al. 1998a; Pierson et al. 1998b) (Figure 1). These AHL signals are used by a diverse range of gram-negative bacteria to regulate the expression of genes involved in both beneficial and detrimental plant-microbe and microbe-microbe interactions.

MODEL FOR AHL GENE REGULATION
AHL-mediated gene regulation is also known as cell density-mediated gene regulation and quorum sensing (QS). There are several excellent reviews on AHL-mediated gene regulation
(Dunlap 1997; Dunny and Winans 1999; Fuqua et al. 2001; Fuqua et al. 1996; Jayaraman and Wood 2008; Ng and Bassler 2009; Papenfort and Bassler 2016; Pierson et al. 1998a; Waters and Bassler 2005). For our purposes, let us consider a simplified model for AHL-mediated gene regulation in bacteria (Figure 2). In this model of QS there are two genes that are important. The first gene encodes a regulatory protein (R) that is required to activate the transcription of the target gene(s). However, the R protein by itself cannot activate expression of the target genes alone (or at least only to very low levels). The second QS gene (I gene) encodes an enzyme known as an AHL synthase that synthesizes a small diffusible AHL signal using the cellular precursors S-adenosyl-methionine (AdoMet) and a fatty acyl ACP
(Moré et al. 1996; Parsek et al. 1999; Schaefer et al. 1996). The AHL signal can diffuse across the bacterial cell wall. How easily is dependent on the length and substitutions of the fatty acyl sidechain.
What is key to remember is that the R protein is active only when it is bound to the AHL signal. At any time when the concentration of AHL signal is low, such as when the cell density of the culture is low, the AHL signal diffuses out of the cell, thus reducing its intracellular concentration below that sufficient to activate the R regulatory protein. When the bacterium is in a confined space or at high cell density, AHL accumulates within the cell, reaching a concentration at which it binds to the R protein (the threshold level). The now activated R regulatory protein binds to specific promoters in the genome of the cell and stimulates (or represses) transcription of the target genes.
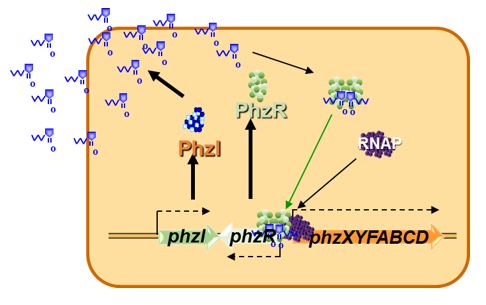
It is important to note that each R regulatory protein recognizes only a subset of possible AHL signals. Thus, failure to activate an AHL reporter strain is not evidence of a lack of AHL production by a bacterium, only that the specific R regulatory protein of the AHL reporter strain does not effectively recognize the signal produced by the other bacterium.
INTERKINGDOM SIGNALING
What is also exciting is that many plants produce signals recognized by specific bacterial QS systems and that plants respond themselves to bacterial signals. For example, pea seedlings can induce pigment production in an AHL reporter strain of
Chromobacterium violacein
(Teplitski et al. 2000). Rice and beans have been shown to produce AHL signal mimics that interfere with colonization of their surfaces by some QS strains of
Sinorhizobium fredii
(Pérez-Montaño et al. 2013). In addition, AHL signals have been shown to induce systemic resistance in tomato plants
(Schuhegger et al. 2006), as well as affect developmental changes and altering architectures of Arabidopsis roots
(Ortíz‐Castro et al. 2008). What this illustrates is another exciting aspect of diffusible signaling-- it can occur within a pure population of a signal strain of bacterium, within a mixed community of bacteria, and between bacteria and their eukaryotic host.
THE BENEFICIAL PSEUDOMONAS STRAIN 30-84
Quorum sensing (QS) systems based on the use of AHL signals have been demonstrated in a number of plant-associated bacteria
(Fuqua et al. 1996; Pierson et al. 1998a; Pierson et al. 1998b; Wood et al. 1997) including
Pseudomonas chlororaphis strain 30-84. This root-colonizing strain is effective as a biological control agent for take-all disease of wheat caused by
Gaeumannomyces graminis var.
tritici (Ggt). Disease suppression results primarily from the production of bacterially-produced secondary metabolites known as phenazines
(Pierson and Thomashow 1992). In addition to their role in disease suppression, phenazines enhance the survival of
P. chlororaphis in the wheat rhizosphere where they have been shown to inhibit some members of the microbial community, stimulate biofilm formation by strain 30-84
(Maddula et al. 2008; Maddula et al. 2006; Mazzola et al. 1992; Wang et al. 2016). Phenazine production in strain 30-84 depends on the expression of the phenazine biosynthetic operon (phzXYFABCDO), which is controlled by the two QS genes
phzI
(Wood and Pierson 1996) and
phzR
(Pierson et al. 1994). What makes this strain a useful reporter for the presence of AHL signals is that one of the major phenazines produced by this strain is bright orange and its production is easily visible without the need for exogenous chromogenic indicators.
(Wood et al. 1997) demonstrated that AHL signals produced by an isogenic donor population restored phenazine gene expression in a
phzI mutant population on wheat and confirmed that AHL signals are shared between populations in the rhizosphere. Subsequent work, in which a rhizobacterial library generated from wheat plants was used, demonstrated that approximately 8% of the rhizosphere bacteria of wheat also positively activated phenazine gene expression via the production of AHL signals
(Pierson et al. 1998b). This phenomenon is called positive cross-communication. In this laboratory exercise, you will screen plant-associated bacteria for their ability to positively cross-communicate with an enhanced AHL reporter strain of
P. chlororaphis.
AN AHL REPORTER STRAIN 30-84I
This strain is a derivative of
P. chlororaphis 30-84 which has been modified to be a useful bioreporter for AHL signals. In strain 30-84I, the
phzI gene (encoding the endogenous AHL synthase) has been inactivated
(Wood and Pierson 1996). Since the accumulation of AHL signal is a requirement for phenazine gene expression, strain 30-84I is unable to produce its endogenous AHL signal and appears white (or lightly orange) due to the lack of activation of the phenazines biosynthetic genes required for the orange pigmentation. In this experiment we will see if some of the other members of the plant microbiome produce AHL signals that can restore the ability of strain 30-84I to produce phenazines, resulting in the production of an orange halo.
Do all bacteria produce AHL signals that restore phenazines production? No, it is a relatively small (ca. 8%) of the other microbiome members that can do this. In addition, some other members of the microbiome may act to block phenazine production by strain 30-84 by producing signals that interfere with normal phenazine QS in strain 30-84. This is called Quorum Sensing Interference (QSI) or negative cross-communication. To examine this possibility, you will also test the effect of the other bacterial microbiome members on the PhzI+ wild type strain 30-84 and look for the inhibition of phenazine production, which is indicated by the absence of orange pigmentation. Previously we found that this is also a small (ca. 7%) of the community
(Morello et al. 2004). I hope that you can already see that
the more strains you test the greater your probability of seeing both positive- and negative cross-communication.
MATERIALS (per group)
-
Selection of recently collected plant roots. Often native grasses are good sources. These should be stored in plastic bags at 4 ºC until use.
-
Vortex mixer
-
Sonicating water bath
-
10-ml tube of sterile phosphate buffered saline (PBS)
-
Sterile 1.5-ml snap-top microfuge tubes (plus containers of open sterile microfuge tubes)
-
Pipettors (P-20 & P-100) and a box of sterile yellow tips
-
6 LB agar plates + Cycloheximide (100 mg/ml). Cycloheximide is an antibiotic that prevents the growth of fungi. We refer to these plates as LB + Cyclo100.
-
3 LB agar plates
-
20-ml LB broth (bottles)
-
Jar of ethanol plus glass spreader
-
Single edge razor blades of small stainless steel scissors
-
Be sure to mark the LB and the LB + Cyclo100 plates differently (e.g. two black stripes and two black stripes plus an orange stripe on the side for LB and LB + Cyclo100, respectively).
-
Multichannel pipettor and sterile tips
-
Replica-pronger (48 pin pattern that matches the microtiter plate pattern)
EXPERIMENTAL PROTOCOL
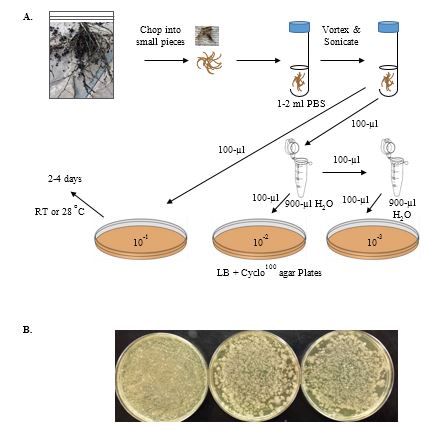
-
Collect several samples of different plants, trying to collect as many of the smaller roots as possible. Store in labeled plastic bags with a slightly damp paper towel at 4 ºC (Figure 3A).
-
Shake off excess soil. You can rinse off the roots with water as what you want are the bacteria tightly associated with the plant surfaces, not the soil or loosely attached bacteria.
-
Take about 1 gram (g) each of your different root materials, and using an ETOH-flamed razor or scissors cut both roots and leaves separately into small pieces with a sterile razor blade. Place the pieces of each sample into separate, sterile glass test tubes. Add 2 ml of sterile PBS buffer to each test tube.
-
Vortex each tube for 30 sec., sonicate 30 sec. Repeat (the sonication step can be omitted but the more tightly adhering bacteria may not be recovered).
-
Serially dilute each to 10-2 in sterile microfuge tubes (each 1/10 dilution is achieved by adding 100-µl sample to 900-µl sterile PBS). Spread 100 µl of each dilution on LB + Cyclo100 plates at 10-1, 10-2, and 10-3. You can store the sonicated suspensions of bacteria at 4
oC for several days. As different plants support different numbers of bacteria (and roots support more than leaves), you may need to plate several plates to obtain a sufficient number of bacterial colonies. Incubate at 28 ºC until isolated colonies of bacteria are easily visible. Check plates daily to see if the numbers of isolated colonies are sufficient. Note that bacteria do not all grow at the same rate, and faster growing bacteria will often overgrow slower growing bacteria. Refrigerate the plates when sufficient growth is observed (Figure 3B).
Next Period: Inoculating cultures for AHL screen plates (Figure 4)
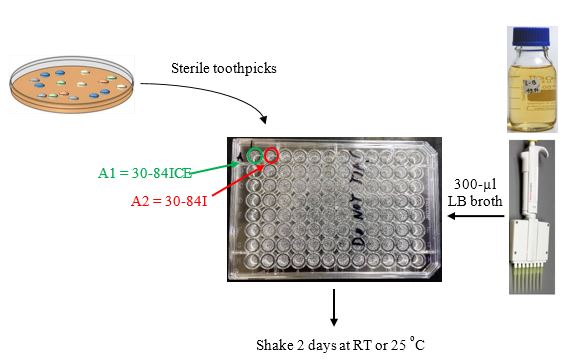
Using sterile toothpicks or sterile loops, pick as many isolated colonies that appear different in shape, color, etc. (this is your test strain collection) and inoculate into 300 µl of LB in 96 well microtiter plates that then are incubated shaking at 28
oC.
Alternative method. An alternative to using a multichannel pipettor and microtiter plates is to have students can toothpick root isolated colonies into small sterile test tubes with LB broth and incubate these with shaking. The limitation of this approach is since the frequency of cross-communication is ca. 5-9%, then many small broth tubes must be inoculated to increase the probability of seeing successful results.
Inoculate control strains.
Inoculate 1-ml LB cultures of strains 30-84, 30-84ICE, & 30-84I. Shake at 28 ºC overnight. Strains 30-84 and 30-84I are the AHL reporter strains, while strains 30-84ICE serves as a positive control strain.
Next Period: Preparation of AHL screen plates (Figure 5)
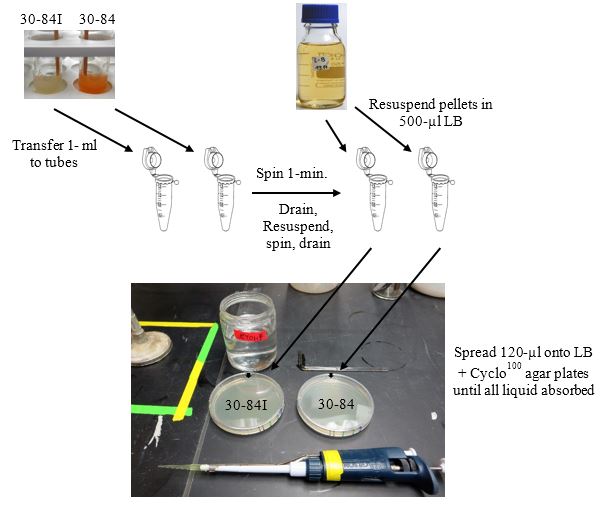
-
Use overnight cultures of strains 30-84I and 30-84 for this step.
-
Aseptically transfer 1-ml of strains 30-84 and 30-84I to separate 1.5 ml microfuge tubes.
-
Microfuge (max. speed) 1-min. Aseptically remove each supernatant.
-
Resuspend each pellet in 500-µl of LB broth using a pipettor with a sterile tip.
-
Microfuge 1-min., and remove supernatant. Resuspend pellet in 500-µl LB.
-
Make an orientation mark on the outside of each plate (best if on the side of the bottom half).
-
Dip a glass spreader in 95% ethanol, pass quickly through a flame, let cool, and spread 120-µl of each culture from step 4 above uniformly onto separate LB agar plates. Continue spreading until all liquid has been absorbed into the plate. These plates are now seeded with a lawn of strain 30-84I or strain 30-84.
Next Periods:
Replica prong test isolate cultures onto seeded lawns of 30-84I and 30-84.
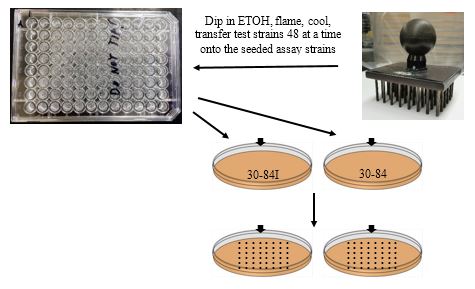
-
Dip the replica-pronger in 95% ETOH and flame. Let cool (or set onto a clean LB agar plate for 5-sec.).
-
Place in first 48 wells and transfer to the seeded lawn of 30-84I.
-
Repeat for the same 48 wells to the 30-84 seeded plate.
-
Let spots soak into the plates before moving the plates. You may store the remaining overnight cultures at 4 ºC for later use.
-
Incubate plates at 28 ºC.
Next Periods (2-3 days): Scoring screening plates for cross-communication (Figure 7).
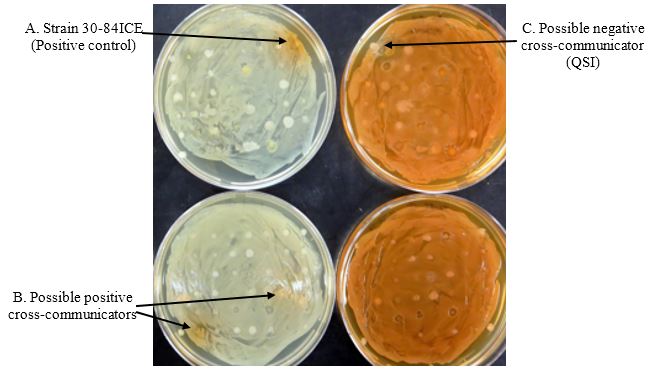
Score the plate seeded with strain 30-84I for the presence of an orange halo surrounding the test spots. The presence of orange halos surrounding the test spots indicates restoration of QS-based phenazine production by a signal produced by the test spots (positive cross-communication) (Figure 7 left side). Score the plate seeded with strain 30-84 for the presence of a white halo surrounding the test spots. The presence of white halos surrounding the test spots indicates inhibition of QS-based phenazine production by strain 30-84 by a signal produced by the test spots (QSI or negative cross-communication) (Figure 7 right side).
You can streak out the positively or negatively cross-communicating strains from the microtiter plate for single colonies on agar medium and repeat the assay or characterize the strains further.
DATA COLLECTION
Set up a score sheet similar to the one illustrated below (Figure 8). Note the plant source (name if known) and number the isolates for each. Score the effect of each isolated test strain on 30-84I or 30-84 by circling the possible positive cross-communicating strains and the possible negative cross-communicating strains.
Plant __________________________ Effect on... |

QUESTIONS FOR THOUGHT
1. What is the percentage of the selected isolates from each plant was capable of cross-communicating with 30-84I? with 30-84? (remember: we only sampled a very small number of possible isolates).
1.A. What would happen if you picked strain 30-84I from the orange halo sector of the plate and re-streaked it onto LB agar by itself? B. Strain 30-84 from the white halo sector and streaked it by itself?
3. Why would different bacteria cross-communicate? That is, list several possible ecological function(s) of cross-communication.
4. Discuss two experiments that you might perform to study the phenomenon of cross-communication further.
5. Does the fact that a test isolate produces an AHL signal
in vitro prove that the same isolate produces an AHL signal
in planta?
6. Some of the test isolates might produce a clear zone in the lawn of
P. chlororaphis. What might this clearing indicate about the interactions between the two strains?
Answers to these discussion questions here.
LITERATURE CITED
Dunlap, P. 1997.
N-Acyl-L-homoserine lactone autoinducers in bacteria: unity and diversity. Pages 69-106 in: Bacteria as multicellular organisms. J. A. Shapiro and M. Dworkin, eds. New York Universtiy Press, New York.
Dunny, G. M., and Winans, S. C. 1999. Bacterial life:Neither lonely nor borning. Pages 1-5 in: Cell-cell signaling in bacteria. G. M. Dunny and S. C. Winans, eds. American Society for Microbilogy Press, Washington, D.C. .
Fuqua, C., Winans, S. C., and Greenberg, E. P. 1996. Census and consensus in bacterial ecosystems: the LuxR-LuxI family of quorum-sensing transcriptional regulators. Annu Rev Microbiol 50:727-751.
Fuqua, C., Parsek, M. R., and Greenberg, E. P. 2001. Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu Rev of Genetics 35:439-468.
Gray, K. M. 1997. Intercellular communication and group behavior in bacteria. Trends Microbiol 5:184-188.
Jayaraman, A., and Wood, T. K. 2008. Bacterial quorum sensing: signals, circuits, and implications for biofilms and disease. Annu. Rev. Biomed. Eng. 10:145-167.
Maddula, V. S., Pierson, E. A., and Pierson, L. S., III. 2008. Altering the ratio of phenazines in
Pseudomonas chlororaphis (aureofaciens) strain 30-84: effects on biofilm formation and pathogen inhibition. J Bacteriol 190:2759-2766.
Maddula, V. S., Zhang, Z., Pierson, E. A., and Pierson, L. S., III. 2006. Quorum sensing and phenazines are involved in biofilm formation by
Pseudomonas chlororaphis (aureofaciens) strain 30-84. Microb Ecol 52:289-301.
Mazzola, M., Cook, R. J., Thomashow, L. S., Weller, D. M., and Pierson, L. S., III. 1992. Contribution of phenazine antibiotic biosynthesis to the ecological competence of fluorescent pseudomonads in soil habitats. Appl Environ Microbiol 58:2616-2624.
Moré, M. I., Finger, L. D., and Stryker, J. L. 1996. Enzymatic synthesis of a quorum-sensing autoinducer through use of defined substrates. Science 272:1655.
Morello, J. E., Pierson, E. A., and Pierson, L. S. I. 2004. Negative cross-communication among wheat rhizosphere bacteria: effect on antibiotic production by the biological control bacterium
Pseudomonas aureofaciens 30-84. Appl Environ Microbiol 70:3103-3109.
Ng, W.-L., and Bassler, B. L. 2009. Bacterial quorum-sensing network architectures. Annu Rev of Genetics 43:197.
Ortíz‐Castro, R., Martínez‐Trujillo, M., and López‐Bucio, J. 2008.
N‐acyl‐L‐homoserine lactones: a class of bacterial quorum‐sensing signals alter post‐embryonic root development in
Arabidopsis thaliana. Plant, cell & environment 31:1497-1509.
Papenfort, K., and Bassler, B. L. 2016. Quorum sensing signal-response systems in Gram-negative bacteria. Nat Rev Microbiol 14:576-588.
Parsek, M. R., Val, D. L., Hanzelka, B. L., Cronan, J. E., and Greenberg, E. 1999. Acyl homoserine-lactone quorum-sensing signal generation. Proc Natl Acad Sci USA 96:4360-4365.
Pérez-Montaño, F., Jiménez-Guerrero, I., Sánchez-Matamoros, R. C., López-Baena, F. J., Ollero, F. J., Rodríguez-Carvajal, M. A., Bellogín, R. A., and Espuny, M. R. 2013. Rice and bean AHL-mimic quorum-sensing signals specifically interfere with the capacity to form biofilms by plant-associated bacteria. Research in Microbiology 164:749-760.
Pierson, L. S., III, and Thomashow, L. S. 1992. Cloning and heterologous expression of the phenazine biosynthetic locus from
Pseudomonas aureofaciens 30-84. Mol. Plant-Microbe Interact 5:330-339.
Pierson, L. S., III, Keppenne, V. D., and Wood, D. W. 1994. Phenazine antibiotic biosynthesis in
Pseudomonas aureofaciens 30-84 is regulated by PhzR in response to cell density. J Bacteriol 176:3966-3974.
Pierson, L. S., III, Wood, D. W., and Pierson, E. A. 1998a. Homoserine lactone-mediated gene regulation in plant-associated bacteria. Annu Rev Phyopathol 36:207-225.
Pierson, L. S., III, Wood, D. W., Pierson, E. A., and Chancey, S. T. 1998b. N-acyl-homoserine lactone-mediated gene regulation in biological control by fluorescent pseudomonads: Current knowledge and future work. Eur J Plant Pathol 104:1-9.
Schaefer, A. L., Val, D. L., Hanzelka, B. L., Cronan, J. E., and Greenberg, E. 1996. Generation of cell-to-cell signals in quorum sensing: acyl homoserine lactone synthase activity of a purified
Vibrio fischeri LuxI protein. Proc Natl Acad Sci USA 93:9505-9509.
Schell, M. A. 2000. Control of virulence and pathogenicity genes of
Ralstonia solanacearum by an elaborate sensory network. Annu Rev Phyopathol 38:263-292.
Schuhegger, R., Ihring, A., Gantner, S., Bahnweg, G., Knappe, C., Vogg, G., Hutzler, P., Schmid, M., Van Breusegem, F., and Eberl, L. 2006. Induction of systemic resistance in tomato by
N‐acyl‐L‐homoserine lactone‐producing rhizosphere bacteria. Plant, Cell & Environment 29:909-918.
Shapiro, J. A. 1998. Thinking about bacterial populations as multicellular organisms. Annu Rev Microbiol 52:81-104.
Teplitski, M., Robinson, J. B., and Bauer, W. D. 2000. Plants secrete substances that mimic bacterial N-acyl homoserine lactone signal activities and affect population density-dependent behaviors in associated bacteria. Mol Plant-Microbe Interact 13:637-648.
Wang, D., Yu, J. M., Dorosky, R. J., Pierson III, L. S., and Pierson, E. A. 2016. The Phenazine 2-Hydroxy-Phenazine-1-Carboxylic Acid Promotes Extracellular DNA Release and Has Broad Transcriptomic Consequences in
Pseudomonas chlororaphis 30–84. PloS one 11:e0148003.
Waters, C. M., and Bassler, B. L. 2005. Quorum sensing: cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 21:319-346.
West, S. A., Griffin, A. S., Gardner, A., and Diggle, S. P. 2006. Social evolution theory for microorganisms. Nat Rev Microbiol 4:597-607.
West, S. A., Diggle, S. P., Buckling, A., Gardner, A., and Griffin, A. S. 2007. The social lives of microbes. Annual Review of Ecology, Evolution, and Systematics:53-77.
Wirth, R. 2000. Sex pheromones and gene transfer in
Enterococcus faecalis. Research in microbiology 151:493-496.
Wood, D. W., and Pierson, L. S., III. 1996. The
phzI gene of
Pseudomonas aureofaciens 30–84 is responsible for the production of a diffusible signal required for phenazine antibiotic production. Gene 168:49-53.
Wood, D. W., Gong, F., Daykin, M. M., Williams, P., and Pierson, L. S., III. 1997. N-acyl
homoserine lactone-mediated regulation of phenazine gene expression by
Pseudomonas aureofaciens 30-84 in the wheat rhizosphere. J Bacteriol 179:7663-7670.
