Introduction
One of the most intriguing classes of plant pathogens are the viroids, subviral pathogens that have been isolated from higher plants afflicted with specific diseases. Viroid hosts include both herbaceous and woody species — agronomic as well as ornamental. Identification of viroid-infected plants can be accomplished by either symptomatology on indicator hosts, a classical method still used in many certification programs, or by molecular methods such as nucleic acid hybridization or polymerase chain reaction.
Several viroid-incited diseases are of considerable economic importance. For example, yield losses can be high in potatoes infected with Potato spindle tuber viroid (PSTVd) (Fig. 1), chrysanthemum infected with Chrysanthemum stunt viroid (CSVd), citrus infected with Citrus exocortis viroid (CEVd), coconut palms infected with Coconut cadang-cadang viroid (CCCVd), and avocado infected with Avocado sunblotch viroid (ASBVd). Exclusion or eradication of infected materials is the most effective means of controlling viroid diseases, but routine diagnosis of viroid diseases based on symptom expression in their natural hosts is often difficult.
| |
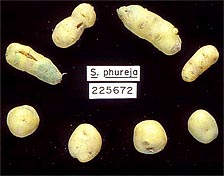
Fig. 1. Potato tubers derived from healthy (bottom) and PSTVd-infected (top) plants. Tuber symptoms include elongation and cracking. |
|
Expression of commonly observed symptoms of viroid infection — stunting, leaf epinasty and distortion, fruit distortion and color break, stem and leaf necrosis, and even death of the whole plant — is often dependent upon environmental conditions. Also, many viruses can cause very similar symptoms. While viroids were initially identified by their association with specific diseases, we now know that not all viroid-infected plants exhibit obvious signs of disease. In some cases, a viroid may be latent in one host yet cause severe symptoms in another host. One example is Columnea latent viroid (CLVd), which was isolated from healthy-looking plants of the ornamental Columnea erythrophae but causes a disease similar to, but more severe than that caused by PSTVd in potato.
Recent outbreaks of diseases caused by viroids suggest that either current control measures are inadequate or that growers may be unaware of the risks that viroid infection pose to their crops. Viroids are also being detected in crop species where they were not previously known to occur. In some cases, these infections are latent, but in others, severe disease symptoms develop. These observations raise several intriguing questions: Why are viroid diseases recurring in certain crops where they were previously assumed to be under control? Are viroid infections more common than we know; if so, do they pose a serious threat to modern agriculture?
Our review of the literature attempts to address these questions. For more photographs of symptoms associated with specific viroid diseases, see Hadidi et al. (16) and the Disease Compendium Series of the American Phytopathological Society for specific crops (APS Press).
Molecular and Biological Features of Viroids
In the late 1960s to early 1970s, the causal agent of potato spindle tuber disease was found to be the first example of a novel class of pathogens — small, single-stranded, covalently-closed-circular RNA molecules that lack a protective protein capsid (Fig. 2). In 1971, Diener renamed the causal agent of spindle tuber disease Potato spindle tuber viroid (PSTVd) to emphasize both its similarities to and differences from conventional viruses (7). Viroid genomes range in size from 239-401 nucleotides and their hosts include both herbaceous and woody plants (Table 1). Unlike plant viruses, viroids apparently lack mRNA activity and, unlike satellite RNAs and DNAs, do not require a helper virus for replication or movement. There are currently 29 known viroid species that are grouped into two families, the Pospiviroidae (type member, PSTVd) and the Avsunviroidae (type member, ASBVd) based on sequence and structural homologies (Table 1). In general, members of the Avsunviroidae have a narrower host range than members of the Pospiviroidae. For a comprehensive description of the host range of viroids, see Hadidi et al. (16).
| |
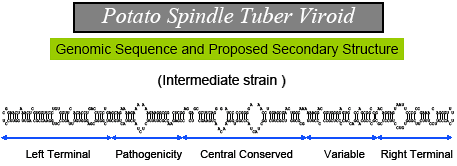
Fig. 2. Genomic sequence and proposed secondary structure of Potato spindle tuber viroid, a covalently-closed circular RNA molecule containing 359 nucleotides. |
|
|
Table 1. Officially recognized viroid species (Eighth Report, International Committee on Taxonomy of Viruses).
| Genusx |
Species |
Variantsy |
Length (nt) |
Natural host(s) |
Emerging hosts |
| Family Pospiviroidae |
| Pospiviroid |
Potato spindle tuber (PSTVd) |
109 |
341-364 |
potato |
tomato, avocado |
| Chrysanthemum stunt (CSVd) |
19 |
348-356 |
chrysanthemum |
|
| Citrus exocortis (CEVd) |
86 |
366-475 |
citrus, tomato |
tomato |
| Columnea latent (CLVd) |
17 |
359-456 |
Columnea, Brunfelsia, Nemathanthus |
tomato |
| Iresine (IrVd) |
3 |
370 |
Iresine |
|
| Mexican papita (MPVd) |
6 |
359-360 |
Solanum cardiophyllum |
|
| Tomato apical stunt (TASVd) |
5 |
360-363 |
tomato |
tomato |
| Tomato chlorotic dwarf (TCDVd) |
2 |
360 |
Uncertain
(tomato?) |
tomato |
| Tomato planta macho (TPMVd) |
2 |
360 |
tomato |
|
| Hostuviroid |
Hop stunt (HSVd) |
144 |
294-303 |
citrus, grapevine, Prunus spp. |
hop, cucumber |
| Cocadviroid |
Coconut cadang-cadang (CCCVd) |
8 |
246-301 |
coconut palm |
African oil palm, other monocots |
| Coconut tinangaja (CTiVd) |
2 |
254 |
coconut palm |
|
| Citrus bark cracking (CBCVd) |
6 |
284-286 |
citrus |
|
| Hop latent (HLVd) |
10 |
255-256 |
hop |
|
| Apscaviroid |
Apple scar skin (ASSVd) |
8 |
329-333 |
apple, pear |
|
| Apple dimple fruit (ADFVd) |
2 |
306 |
apple |
|
| Apple fruit crinkle (AFCVd)z |
29 |
368-372 |
apple |
|
| Australian grapevine (AGVd) |
1 |
369 |
grapevine |
|
| Citrus bent leaf (CBLVd) |
24 |
315-329 |
citrus |
|
| Citrus dwarfing (CDVd) |
53 |
291-297 |
citrus |
|
| Grapevine yellow speckle 1 (GYSVd-1) |
49 |
365-368 |
grapevine |
|
| Grapevine yellow speckle 2 (GYSVd-2) |
1 |
363 |
grapevine |
|
| Pear blister canker (PBCVd) |
18 |
314-316 |
pear, quince |
|
| Coleviroid |
Coleus blumei-1 (CbVd-1) |
9 |
248-251 |
Coleus,
Mentha spp. |
|
| Coleus blumei-2 (CbVd-2) |
? |
295-301 |
|
|
| Coleus blumei-3(CbVd-3) |
3 |
361-364 |
Ocimum basilicum, Melissa officinalis |
|
| Family Avsunviroidae |
| Avsunviroid |
Avocado sun blotch (ASBVd) |
83 |
239-251 |
avocado |
|
| Pelamoviroid |
Chrysanthemum chlorotic mottle (CChMVd) |
21 |
397-401 |
chrysanthemum |
|
| Peach latent mosaic (PLMVd) |
168 |
335-351 |
peach, nectarine |
|
| Elaviroid |
Eggplant latent (ELVd) |
9 |
332-335 |
eggplant |
|
x Names of viroid genera are derived from those of the respective type species (listed first).
y Sequences available online from the Subviral RNA Database [http://subviral.med.uottawa.ca].
z Provisional species (not officially recognized). |
Pospiviroids replicate in the nucleus and accumulate in the nucleolus, whereas avsunviroids have been shown to accumulate, and presumably replicate, in the chloroplast. Both employ a rolling-circle type of replication using different host DNA-dependent RNA polymerases. Systemic infection of the host plant involves both movement from cell to cell through the plasmodesmata and long distance movement paralleling the transport of photosynthate. Research is currently underway to identify host/pathogen interactions that facilitate the movement of viroids in plants and that result in disease induction [reviewed by Diener, (9), Flores et al. (12) and Ding et al. (10)].
The Role of Mechanical Contact, Seed Transmission, and Biological Vectors in Viroid Spread
Field and greenhouse studies have demonstrated that viroids are easily transmissible by mechanical inoculation and efficiently spread by contact with contaminated pruning tools, farm implements, clothing, and human hands. Viroids can also be spread by graft transmission and foliar contact between neighboring plants.
Seed transmission has been demonstrated for many, but not all, viroids, and pollen-borne transmission is also known to occur in tomato (25). For potatoes, PSTVd may be introduced into a field by planting infected seed tubers or true potato seed. Reports of seed transmission of CSVd are contradictory, with Monsion et al. (28) presenting evidence for seed transmission, whereas Hollings and Stone (20) report that CSVd is not seed transmitted. Vertical transmission has been demonstrated for ASBVd in avocado (47) and PSTVd in tomato and pepino (2,20,42) but not Tomato planta macho viroid (TPMVd) in tomato (14).
Insect transmission was reported for TPMVd, where the aphid, Myzus persica was found to transmit the viroid from the wild host, Physalis foetens, to the experimental host, tomato. PSTVd can also be transmitted by insect vectors. De Bokx and Pirone (6) reported a low rate of transmission of PSTVd by the aphid Macrosiphum euphorbiae but not by Myzus persicae or Aulacorthum solani. However, Myzus persicae is able to efficiently transmit PSTVd from plants that are doubly-infected with PSTVd and potato leafroll luteovirus (PLRV) (36). PSTVd was subsequently shown to be heterologously encapsidated within particles of PLRV (34), a phenomenon that may have important implications for the epidemiology and spread of PSTVd under field conditions.
Diagnostic Methods

Fig. 3. Rutgers tomato plants infected with naturally occurring strains of Potato spindle tuber viroid exhibit mild to severe symptoms of stunting, epinasty, and leaf rugosity.
|
|
As stated earlier, viroids can be detected by biological indexing (bioassay) of the suspect plant materials on a range of indicator hosts. Indicator hosts express diagnostic symptoms when infected by specific pathogens (Fig. 3). For biological indexing to be successful, both the host range as well as the symptoms produced on that host by specific viroids must be known.
The next level of sophistication in viroid diagnosis depends on knowledge of their molecular properties. As viroids contain neither capsid nor nonstructural proteins, ELISA is not an option. Early molecular detection methods involved a combination of native and denaturing polyacrylamide gel electrophoresis that relies on the circular properties of the viroid RNA molecule to resolve it from other plant RNAs (41,43) (Fig. 4). These methods are still very useful for demonstrating the presence of new viroids in diseases of unknown etiology, but they are not as useful for the identification of specific viroids, as several viroids may have the same electrophoretic mobility.
| |
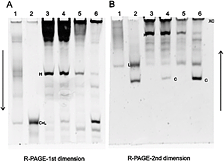
Fig. 4. Detection of Potato spindle tuber viroid by return polyacrylamide electrophoresis. (A) first dimension (non-denaturing) and (B) second dimension (denaturing). C, L - circular and linear forms of the viroid molecule; H - host RNA; XC-xylene cyanol marker dye. |
|
Owens and Diener (31) were the first to demonstrate the use of molecular hybridization for viroid detection (Fig. 5A). Although reliable for viroids of known sequence and very sensitive, this method cannot be relied upon to detect new viroids where sequence information is unavailable. Recently, a number of reverse transcription-polymerase chain reaction (RT-PCR) protocols have been developed for detection of different viroids (Fig 5B), including real-time RT-PCR (3). In many cases, PCR complements biological indexing; it is especially useful for identifying symptomless viroid carriers. For example, using pospiviroid primers based on conserved and viroid-specific sequences, Verhoeven et al. (46) identified several viroids in natural infections of tomato — two of which had not been previously reported in this crop. Although rapid and very sensitive, RT-PCR methods rely upon sequence knowledge, are sensitive to single mutations (and therefore may not detect all strains), and are expensive. The future of viroid detection may be the utilization of DNA microarrays that can simultaneously detect and identify multiple plant pathogens based on oligonucleotides representing known target sequences (15).
| |
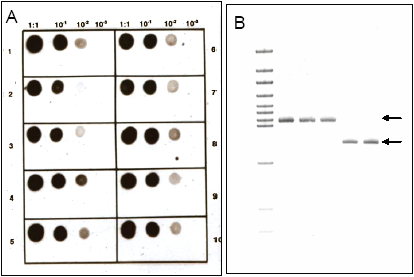
Fig. 5. Viroid detection by dot blot hybridization using a non-radioactive nucleic acid probe and nylon filters spotted with total nucleic acids extracted from leaf tissue (A), or RT-PCR using two sets of viroid-specific oligonucleotide primers followed by analysis of RT-PCR products (designated by arrows) using polyacrylamide gel electrophoresis (B). |
|
Control of Viroid Diseases
As mentioned in the Introduction, the most effective means for control of viroid diseases is exclusion or eradication of infected materials. Phytosanitary regulations and policies established by individual countries or organizations are designed to prevent the entry and spread of pests, and the diagnostic methods just described are invaluable tools that aid in identification of viroid-infected plants. Some infected material is simply too valuable to be discarded, however. Viroids infecting fruit trees can be eliminated by thermotherapy and meristem culture, thereby providing a source of viroid-free propagation material [for example, ASSVd in apple (21)]. Viroid-free chrysanthemum plants have also been obtained by meristem-tip culture after heat treatment (19). Alternatively, cold treatment has also been used for elimination of ASSVd from infected pear plants (32). Control measures based on biotechnology, including creation of viroid-resistant plants using transgenic technology, are currently being developed but are not yet employed in the field (38,40).
Appearance and Reappearance of Viroid Infections in Specific Crops
Routine indexing of diagnostic samples has revealed that several viroids are more widely distributed than previously thought. There are lessons to be learned from both the discovery of novel viroids and the rediscovery of known viroids in new crop plants that can help prevent the outbreak of new infections.
Herbaceous ornamentals: The importance of symptomless reservoirs. Columnea latent viroid was discovered by John Randles and T.O. Diener during a search for alternate, herbaceous hosts of Coconut cadang cadang viroid (CCCVd). CCCVd was mechanically inoculated onto ornamental plants obtained from a commercial nursery, and nucleic acid extracts from the inoculated leaves were then mechanically inoculated onto leaves of Rutgers tomato plants. Quite unexpectedly, symptoms similar to, but less severe than, those produced by a severe strain of PSTVd, were observed on tomatoes inoculated with extracts prepared from apparently healthy Columnea erythrophae control plants. Symptoms in potato were also typical of those incited by PSTVd.
This new viroid was designated Columnea latent viroid, and its nucleotide sequence exhibits similarities to several members of the pospiviroid group (17). C. erythrophae is an epiphyte from Central America, and although no viroids could be isolated from over one hundred samples of Columnea spp. collected in Costa Rica, sampling of selected cultivars obtained from European nurseries did reveal the presence of a CLVd-related RNA. The origin of CLVd remains unknown, but its discovery represents the first case where an apparently healthy ornamental species was shown to harbor a viroid capable of causing disease in an agricultural crop.

Fig. 6. Coleus blumei sown from seed contains a mixture of uninfected plants and plants infected with CbVd. Plants containing CbVd are symptomless carriers of the pathogen.
|
|
Several years later, Fonseca et al. (13) discovered another new viroid in field-grown yellow Coleus in Brazil. Subsequent studies have shown that Coleus blumei viroid (CbVd) is widely distributed throughout the world and often symptomless (Fig. 6). There are at least three different species of CbVd, and RNA recombination appears to play an important role in generating sequence diversity. Fortunately, the host range of CbVd appears to be rather restricted and does not include any major crop species.
A third viroid, Eggplant latent viroid (ELVd), was discovered by Fagoaga et al. (11) during a survey of viroid and viroid-like RNAs in vegetable crops grown in eastern Spain. This avsunviroid which was initially identified in symptomless plants of the eggplant cultivar ‘Sonja’ was shown to be mechanically transmissible to other cultivars by razor blade slashing. Efforts to transmit ELVd to tomato, cucumber, chrysanthemum, and citron were unsuccessful.
Most recently, a survey of ornamental plants by Bostan et al. (5) revealed the frequent presence of CEVd in Impatiens spp. and CSVd in Verbena and Vinca spp., indicating widespread contamination of ornamentals with pospiviroids of agronomic importance. Similar to the situations described above, infected plants were symptomless and, thus, may serve as potential pospiviroid reservoirs. Ornamentals may also serve as a means of introduction of viroids to other greenhouse crops such as tomato, for it is not uncommon for transplants to be raised in the same glasshouse as ornamentals (see below).
Woody perennials: A possible ancient origin for certain viroid diseases and the dangers associated with germplasm exchange.
Pome and stone fruits. Pome and stone fruits, widely grown in temperate climates around the world, are susceptible to a number of viroid diseases. Several of the viroids involved belong to the genus Apscaviroid (see Table 1). Apple scar skin viroid (ASSVd), type member of this genus, is widespread in apples grown in East Asia but rare in North America and Europe. ASSVd infection of pear is also widespread in China and Greece; in Greece, high rates of infection have been reported for wild pear that is widely used as rootstock.
Peach latent mosaic viroid (PLMVd) and Hop stunt viroid (HSVd) are not Apscaviroids, but these two viroids also threaten fruit production. Very common in peach and nectarine, PLMVd has also been isolated from a variety of other Prunus spp. HSVd causes serious dapple fruit diseases in both plum and peach; symptomless hosts include apricot and almond. A large number of HSVd sequence variants have been described (see Table 1), and phylogenetic analysis suggests that these variants can be divided into several clusters depending on the original host species (24,39). Based on this information, Sano et al. (39) have proposed that hop stunt disease, which is only found in Japan, originated sometime in the early 20th century following a chance transfer of HSVd from grapevine to hop.
Molecular methods (dot blot hybridization or RT-PCR) have been developed to detect most of the viroids that infect pome and stone fruits, and quarantine regulations in most countries require that imported germplasm be tested for their presence. Also, many of these viroids can be successfully eliminated by a combination of thermotherapy and shoot tip grafting/meristem culture. These control efforts are complicated by several factors; among the most important are (i) an absence of conspicuous symptoms or their restriction to a single tissue (e.g., the fruit) and (ii) the prevalence of grafting and other sorts of cutting and pruning operations. In Japan, top-working has been implicated in the spread of Apple fruit crinkle viroid (AFCVd).
Citrus and grapevine. Rather than consequences of 20th century agricultural technology, certain viroid diseases (and especially those affecting citrus) may actually be quite ancient in origin. As discussed by Bar-Joseph (1), grapevines and fruit trees are known to contain a variety of different viroids and have been cultivated in the Middle East and Asia for millennia. Citrus, in contrast, is believed to have been introduced from the Himalayas at a much later date. Grapevines harbor several of the same viroids currently found in citrus (i.e., CEVd, HSVd, and several apscaviroids), but these viroids are not seed transmitted in citrus. Because citrus was almost certainly transported to the Middle East as seed, viroid infection probably occurred after arrival and planting next to infected (but symptomless) grapevines. Archaeological evidence supporting this scenario has been summarized by Bar-Joseph (1). According to Bar-Joseph, certain Jewish farming practices (‘Rules of Conduct’ that prohibit the close cultivation of non-related annual crops in vineyards and apparently designed to enforce grapevine monoculture) may also have acted as phytosanitary measures to prevent the outbreak of diseases spread by cultivation tools, contact, and heterologous grafting.
In the 1930s and 1940s, the Australian citrus industry was decimated by Phytophthora root rot affecting trees grafted on sweet orange or rough lemon root stocks. The only resistant rootstock then available was trifoliate orange [Poncirus trifoliate (L.) Raf.], but some cultivars on this rootstock were dwarfed and unthrifty, with obvious bark scaling near the graft union. Also known as "exocortis" in the U.S., these bark scaling symptoms signal the presence of CEVd in the latently infected scion. A similar situation has arisen more recently in Florida where the arrival of brown citrus aphid from the Caribbean has necessitated the replacement of sour orange (a widely used and viroid-tolerant rootstock) with trifoliate orange and trifoliate hybrids (resistant to Citrus tristeza virus ‘quick decline’, but sensitive to viroid infection). Although viroids cause several different diseases in citrus [e.g., cachexia and xyloporosis (HSVd) in addition to exocortis (CEVd)], it is important to note that viroid infection can also be beneficial to citrus production. For example, both CEVd and Citrus dwarfing viroid (formerly known as Citrus viroid IIII) have been used to dwarf citrus, thereby allowing a predictable degree of tree size control and the use of higher planting densities [reviewed by Hutton et al. (23)].
Coconut and oil palm. The lethal effect of Coconut cadang-cadang viroid (CCCVd) and Coconut tinangaja viroid (CTiVd) on coconut palms growing in the Philippines and Guam has long been recognized, but the distribution of these two viroids was considered to be restricted to their respective geographic regions. A series of studies carried out by John Randles and colleagues and dating back to the early 1980s now indicates that infection by CCCVd-related viroids may be much more widespread than previously believed [reviewed by Hanold and Randles (18)]. In particular, the well-known orange leaf spotting disorder of oil palm now appears to be infectious rather than ‘genetic’ in nature.
Hybridization analyses carried out under low stringency conditions have demonstrated the presence of a number of small CCCVd-related RNAs in both palm and other monocot species. In oil palm, the presence of these RNAs is strongly correlated with the presence of ‘genetic orange spotting’ symptoms. The high incidence of CCCVd-related RNAs in the Thailand-Malaysia-West Indonesia area is of particular concern, because this is the region from which many of the dwarf lines used for breeding originate. Movement of coconut germplasm by breeders is extensive and, in the absence of conclusive evidence for their infectivity, quarantine measures based on the presence of these CCCVd-related RNAs remains controversial.
Tomato: Have modern agricultural practices accidentally created a "viroid sink"? Although tomato is a widely used experimental host for pospiviroids [see Table 15.1 in (45)], only three of these viroids [i.e., Indian Tomato bunch top viroid (a strain of CEVd), Tomato apical stunt viroid (TASVd), and TPMVd] have been isolated from field-grown tomatoes. Three others [i.e., Mexican papita viroid (MPVd), Tomato chlorotic dwarf viroid (TCDVd), and CLVd] were detected in plants growing in commercial greenhouses. As described above, PSTVd has also been isolated from greenhouse-grown tomatoes on several occasions, and it is possible that the uncharacterized agent responsible for outbreaks of "tomato bunchy top disease" in South Africa more than 50 years ago may have been a severe strain of PSTVd.
In the Netherlands, the inoculum source for a late 1980s outbreak of PSTVd in greenhouse-grown tomatoes is thought to have been infected pepino seed imported from New Zealand and Greece (33). Similarly, TCDVd was originally detected in Canada in tomatoes grown from seed imported from the Netherlands via the US (44). A common thread connecting these outbreaks is the ability of each of these viroids to cause disease in at least one important crop species (tomato) while all but TCDVd are known to have at least one asymptomatic host. Seed transmissibility also appears to be an important factor.
Potato: The need for continued vigilance. PSTVd is classified as both an European Union Annex 1/A1 and an European and Mediterranean Plant Protection Organization (EPPO) A2 quarantine organism (30). This means that seed potatoes must be certified as free of PSTVd before export, thereby requiring rigorous testing of plant material. Because of this certification requirement, PSTVd has virtually been eliminated from potato production areas in the USA, Canada, and Australia because it is no longer introduced into the field each growing season via contaminated seed potato. If it were to be re-introduced, however, spread could be rapid.
PSTVd is readily transmitted mechanically as well as through botanical seed and pollen of tomato and potato. Bonde and Merriam (4) found that contaminated cutting knives used for tuber propagation resulted in the highest percentages of infection. Merriam and Bonde (27) reported 80 to 100% field transmission of PSTVd to healthy potato plants when tractor wheels became contaminated with infectious sap due to bruising of diseased potato plants. Similarly, Manzer and Merriam (26) found nearly 100% field transmission of PSTVd to healthy potato plants when tractor wheels became contaminated with infectious sap due to bruising of diseased potato plants. Similarly, Manzer and Merriam (25) found nearly 100% transmission with cultivating and hilling equipment, where large plants were present, and very little transmission when the plants were smaller and when cultivation was performed earlier in the season.
Although the primary natural host of PSTVd is potato, this viroid also naturally infects a variety of other Solanum spp. Its experimental host range also includes a wide range of species from other families including sweet potato (Ipomoea batatas). In Peru, PSTVd has been detected in avocado (Persea americana), where infections are often latent unless the tree is co-infected with ASBVd (35). More recently, PSTVd has been reported to be associated with a new disease of glasshouse tomato and Capsicum crops in New Zealand (Crop & Food Research, Number 139). Interestingly, potatoes growing nearby appear to have been uninfected. A similar outbreak of PSTVd was reported in a tomato production nursery in the south-east of England (29). The origin of these infections is currently under investigation.
Conclusions and Perspectives
The origin of viroids is unknown (8), but man has clearly played a central role in creating and perpetuating the diseases they cause. When compared to diseases known to be caused by viruses, many viroid diseases appear to be relatively recent in origin — potato spindle tuber disease was first reported in 1922, chrysanthemum stunt disease in 1947, and cucumber pale fruit in 1974. The prevalence of latent infections provides one explanation for the comparatively recent discovery of viroids; i.e., wild plants or symptomless carriers act as reservoirs, and it is only the rare chance transfer to susceptible cultivated plants (e.g., potato, tomato) that results in disease. The modern emphasis on monoculture of genetically identical crops, commercial propagation, and worldwide distribution of improved varieties increases the likelihood that a chance infection will lead to the development of a disease epidemic and the attendant crop losses. As suggested by archeological evidence for the transfer of viroids from grapevine into citrus nearly two thousand years ago (1), however, very little under the sun turns out to be really new.
Molecular detection methods have clearly improved our ability to detect and eliminate viroid-infected plants, yet new disease outbreaks continue to be reported. Questions which arise and need to be addressed if new outbreaks are to prevented include: Have there been certain changes in agricultural/greenhouse practices (e.g., growing multiple crops that may be symptomless carriers next to susceptible crops) that can explain the appearance of new infections? Should these practices be modified to avoid chance infections of cultivated crops?
Bar-Joseph (1) suggests that for woody perennials, old vegetatively-propagated crops should be located and evaluated as possible symptomless carriers and sources of viroids capable of causing disease in newly planted cultivars. In addition, deliberate evaluation of known viroids in combination with different rootstocks would also be valuable as changing rootstocks is often the growers response to other (fungal, viral) disease pressures. Roguing of these plants would prevent reintroduction of viroid infections. In the absence of either conventional genetic resistance to viroid infection or commercially available engineered resistant cultivars, the best means for control of viroid diseases is grower vigilance, the use of stringent hygiene procedures, and monitoring the crop for unusual symptoms.
Acknowledgments
We thank J. Th. J. Verhoeven for sharing information and insights on seed transmission of viroids and the incidence of viroid infections in tomato crops. We also thank John Hammond and Ed Dougherty for critical reading of the manuscript.
Disclaimer
Mention of a trademark, warranty, proprietary product or vendor does not constitute a guarantee by the United States Department of Agriculture and does not imply its approval to the exclusion of other products or vendors that may also be suitable.
Additional Resources
Subviral RNAs:
penelope.med.usherb.ca/subviral
ICTVdB Index of Viruses:
www.danforthcenter.org/iltab/ictvnet/asp/iVirusIndex.asp
Protocol for diagnosis of a quarantine organism PSTVd:
www.csl.gov.uk/science/organ/ph/diagpro
Molecular Plant Pathology Laboratory:
www.ars.usda.gov/Main/site_main.htm?modecode=12-75-25-00
Literature Cited
1. Bar-Joseph, M. 2003. Natural history of viroids - horticultural aspects. Pages 246-251 in: Viroids. A. Hadidi, R. Flores, J. W. Randles, and J. S. Semancik, eds. CSIRO Publishing, Collingwood, Australia.
2. Benson, A. P, and Singh, R. P. 1964. Seed transmission of potato spindle tuber virus in tomato. Am. Potato J. 41:294.
3. Boonham, N., González-Pérez, L., Mendez, M. S., Lilia Peralta, E., Blockley, A., Walsh, K., Barker, I., and Mumford, R. A. 2004. Development of a real-time RT-PCR assay for the detection of Potato spindle tuber viroid. J. Virol. Methods 116:139-146.
4. Bonde, R., and Merriam, D. 1951. Studies on the dissemination of the potato spindle tuber virus by mechanical inoculation. Am. Potato J. 28:558–560.
5. Bostan, H., Nie, X., and Singh, R. P. 2004. An RT-PCR primer pair for the detection of Pospiviroids and its application in surveying ornamental plants for viroids. J. Virol. Methods 116:189-103.
6. De Bokx, J. A., and Pirone, P. G. 1981. Transmission of potato spindle tuber viroid by aphids. Neth. J. Plant Pathol. 87:31.
7. Diener, T. O. 1971. Potato spindle tuber "virus". IV. A replicating, low molecular weight RNA. Virology 45:411-428.
8. Diener, T. O. 2001. The viroid: biological oddity or evolutionary fossil? Adv. Virus Res. 57:137-184.
9. Diener, T. O. (ed). 1987. The Viroids. Plenum Press, New York, NY.
10. Ding, B., Itaya, A., and Zhong, X. 2005. Viroid trafficking: A small RNA makes a big move. Curr. Opin. Plant Biol. 8:606-612.
11. Fagoaga, C., Pina, J. A., and Duran-Vila, N. 1994. Occurrence of small RNAs in severely diseased vegetable crops. Plant Dis. 78:749-753.
12. Flores, R., Hernandez, C., Martinez de Alba, A. E., Daros, J. A., and Di Serio, F. 2005. Viroids and viroid-host interactions. Ann. Rev. Phytopathol. 43:117-139.
13. Fonseca, M. E. N., Boitteux, L. S., Singh, R. P., and Kitajima, E. W. 1989. A small viroid in Coleus species from Brazil. Fitopatol. Bras. 14:94-96.
14. Galindo, J. A. 1987. Tomato plant macho. Pages 315-320 in: The Viroids. T. O. Diener, ed. Plenum Press, New York, NY.
15. Hadidi, A., Czosnek, H., and Barba, M. 2004. DNA microarrays and their potential applications for the detection of plant viruses, viroids, and phytoplasmas. J. Plant Path. 86:97-104.
16. Hadidi, A., Flores, R., Randles, J. W., and Semancik, J. S., eds. 2003. Viroids. CSIRO Publishing, Collingwood, Australia.
17. Hammond, R., Smith, D. R., and Diener, T. O. 1989. Nucleotide sequence and proposed secondary structure of Columnea latent viroid: A natural mosaic of viroid sequences. Nuc. Acids Res. 17:10083-10094.
18. Hanold, D., and Randles, J. W. 2003. CCCVd-related molecules in oil palms, coconut palms and other monocotyledons outside the Phillipines. Pages 336-340 in: Viroids. A. Hadidi, R. Flores, J. W. Randles, and J. S. Semancik, eds. CSIRO Publishing, Collingwood, Australia.
19. Hollings, M., and Stone, O. M. 1970. Attempts to eliminate chrysanthemum stunt from chrysanthemum by meristem-tip culture after heat treatment. Ann. Appl. Biol. 65:311-315.
20. Hollings, M., and Stone, O. M. 1973. Some properties of chrysanthemum stunt, a virus with the characteristic of an uncoated ribonucleic acid. Ann. Appl. Biol. 74:333-348.
21. Howell, W. E., Burgess, J., Mink, G. I., Skrzeczkowski, L. J., and Zhang, Y. P. 1998. Elimination of apple fruit and bark deforming agents by heat therapy. Acta Hortic. 472:641-646.
22. Hunter, D. E., Darling, H. M., and Beale, W. L. 1969. Seed transmission of potato spindle tuber virus. Am. Potato J. 46:247-250.
23. Hutton, R. J., Braodbent, P., and Bevngton, K. B. 2000. Viroid dwarfing for high density citrus plantings. Hort. Rev. 24:277-317.
24. Kofalvi, S. A., Marcos, J. F., Canizares, M. C., Pallas, V., and Candressse, T. 1997. Hop stunt viroid (HSVd) sequence variants from Prunus species: evidence for recombination between HSVd isolates. J. Gen. Virol. 78:3177-3186.
25. Kryczynski, S., Paduch-Cichal, E., and Skzeczkowski, L. J. 1988. Transmission of three viroids through seed and pollen of tomato plants. J. Phytopathol. 121:51-57.
26. Manzer, F. E., and Merriam, D. 1961. Field transmission of potato spindle tuber virus and virus X by cultivating and hilling equipment. Am. Potato J. 38:346-352.
27. Merriam, D., and Bonde, R. 1954. Dissemination of spindle tuber by contaminated tractor wheels and by foliage contact with diseased plants. Phytopathology 44:111.
28. Monsion, M., Bachelier, J.-C., and Dunez, J. 1973. Quelques propriétés d’un viroїde: le rabougrissement du chrysanthème. Ann. Phytopathol. 5:467-469.
29. Mumford, R. A., Jarvis, B., and Skelton, A. 2004. The first report of Potato spindle tuber viroid (PSTVd) in the UK. Plant Path. 53:242.
30. OEPP/EPPO. 1978. Data sheets on quarantine organisms. No. 97. Potato spindle tuber viroid. OEPP/EPPO Bull. 8:2.
31. Owens, R. A., and Diener, T. O. 1981 Sensitive and rapid diagnosis of potato spindle tuber viroid disease by nucleic acid hybridization. Science 213:670-672.
32. Postman, J. D., and Hadidi, A. 1995. Elimination of apple scar skin viroid from pears by in vitro thermo-therapy and apical meristem culture. Acta Hortic. 386:536-543.
33. Puchta, H., Herold, T., Verhoeven, K., Roenhorst, A., Ramm, K., Schmidt-Puchta, W., and Sanger, H. L. 1990. A new strain of potato spindle tuber viroid (PSTVd-N) exhibits major sequences differences as compared to all other PSTVd strains sequenced so far. Plant Mol. Biol. 15:509-511.
34. Querci, M., Owens, R. A., Bartolini, I., Lazarte, V., and Salazar, L. F. 1997. Evidence for heterologous encapsidation of potato spindle tuber viroid by potato leafroll virus. J. Gen. Virol. 78:1207-1211.
35. Querci, M., Owens, R. A., Vargas, C., and Salazar, L. F. 1995. Detection of potato spindle tuber viroid in avocado growing in Peru. Plant Dis. 79:196-202.
36. Salazar, L. F., Querci, M., Bartolini, I., and Lazarte, V. 1995. Aphid transmission of potato spindle tuber viroid assisted by potato leafroll virus. Fitopathologia 30:50-58.
37. Sano, T. 2003. Hop stunt viroid. Pages 207-212 in: Viroids. A. Hadidi, R. Flores, J. W. Randles, and J. S. Semancik, eds. CSIRO Publishing, Collingwood, Australia.
38. Sano, T., Nagayama, A., Ogawa, T., Ishia, I., and Okada, Y. 1997. Transgenic potato expressing a double-stranded RNA-specific ribonuclease is resistant to potato spindle tuber viroid. Nature Biotech. 15:1290-1294.
39. Sano, T., Mimura, R., and Ohshima, K. 2000. Phylogenetic analysis of hop stunt viroid supports a grapevine origin for hop stunt disease. Virus Genes 22:53-59.
40. Sano, T., Hammond, R. W., and Owens, R. A. 2003. Biotechnological approaches for controlling viroid diseases. Pages 343-349 in: Viroids. A. Hadidi, R. Flores, J. W. Randles, and J. S.Semancik, eds. CSIRO Publishing, Collingwood, Australia.
41. Schumacher, J., Meyer, N., Riesner, D., and Weideman, H. L. 1986. Diagnostic procedure for detection of viroids and viruses with circular RNAs by ‘return’-gel electrophoresis. J. Phytopathol. 115:332-343.
42. Singh, R. P. 1970. Seed transmission of potato spindle tuber virus in tomato and potato. Am. Potato J. 47:225-227.
43. Singh, R. P., and Boucher, A. 1987. Electrophoretic separation of a severe from mild strains of potato spindle tuber viroid. Phytopathology 77:1588-1591.
44. Singh, R. P., Nie, X., and Singh, M. 1999. Tomato chlorotic dwarf viroid: an evolutionary link in the origin of pospiviroids. J. Gen. Virol. 80:2823-2328.
45. Singh, R. P., Ready, K. F. M., and Nie, S. 2003. Viroids of solanaceous species. Pages 125-136 in: Viroids. A. Hadidi, R. Flores, J. W. Randles, and J. S. Semancik, eds. CSIRO Publishing, Collingwood, Australia.
46. Verhoeven, J. Th. J., Jansen, C. C. C., Willemen, T. M., Kox, L. F. F., Owens, R. A., and Roenhorst, J. W. 2004. Natural infections of tomato by Citrus exorcortis viroid, Columnea latent viroid, Potato spindle tuber viroid and Tomato chlorotic dwarf viroid. Eur. J. Plant Path. 110:823-831.
47. Whitesell, R. 1952. Sunblotch disease of avocado. Calif. Avocado Soc. Yearbook 37:216.
