Peer-Reviewed by Plant Health Progress
Accepted for publication 27 August 2001. © 2001 Plant Health Progress.
Michelle Marvier, Department of Biology and Environmental Studies Institute, Santa Clara University, Santa Clara, CA 95053
Corresponding author: Michelle Marvier. mmarvier@scu.edu
Marvier, M. 2001. Can risk analysis “colorize” the black and white of transgenic crops? Online. Plant Health Progress doi:10.1094/PHP-2001-0831-01-RV.
Introduction

Fig. 1. Because rice is a major staple food, improvements in the nutritional quality of rice could elevate the health status of millions of people worldwide (click image for larger view). |
|
That “golden rice,” created by the fusion of daffodil genes into the DNA of rice, could someday prevent 1 million to 2 million deaths of children each year made headlines around the world (Fig. 1) (29). Golden rice has become the poster child for all the good that could come of biotechnology -- this nutritionally enhanced plant, with elevated beta-carotene and iron (25,45), has done much to improve the public image of genetically engineered crops. But the headlines made by transgenic crops are not always so uplifting. For example, in June 2001 a major research lab at the University of Washington was burned down by ecoterrorists who had targeted the facility because of research concerning transgenic trees that was only weakly associated with the lab. Meanwhile, the public is confused and largely unaware of just how widespread are applications of biotechnology to agriculture. In a recent survey 34% of Americans were not even aware that many of the foods found on their grocery shelves already contain products from transgenic plants (1).
Debates about new technologies such as genetic engineering are neither new nor surprising. They arise from conflicting values and priorities, from perceptions of past technical abuses, and from rational concerns. Although the polarization of these debates is often blamed on the environmentalist or anti-technology side, in fact the attitudes of pro-technology stakeholders also contribute to the polarization (3). A relatively new scientific field called “risk analysis” has been established in recent decades to help society and decision-makers regulate technology in a way that balances risks and benefits, and mutes the sorts of exaggerations used to support acts of ecoterrorism or claims that biotechnology will feed the world.
Approaches to risk analysis for transgenic crops are still under development, but the hope is that scientifically-based risk analysis of these crops will help this new technology to develop along paths most beneficial to society. In this paper I briefly review just where we are in terms of agricultural biotechnology, and then focus on the ecological (as opposed to human health) risks of these crops. It is a political and regulatory reality that risk analysis has become as much a part of the development of new technology as the technology itself. In reviewing the environmental risks associated with genetically engineered crop plants, I suggest how better risk analysis could promote a more rational discussion regarding biotechnology. I end the paper, however, with some sobering reminders that no amount of risk analysis can eliminate the opposition by some to biotechnology. Although as scientists we like to reduce all debates to technical exchanges and statistical probabilities, much of the resistance to biotechnology reflects cultural, philosophical or even religious values (none of which comfortably factor into risk analysis equations).
Genetically Engineered Crops of Today and Tomorrow
Genetic engineering is a suite of molecular techniques that allows the transfer of genes from any species into the genome of virtually any other species, no matter how unrelated the two species might be. Consequently, organisms with entirely new combinations of properties can be created. Transgenic plant varieties that might be commercialized in the foreseeable future include plants that accumulate toxins and could be used in phytoremediation, oilseed rape and other plants that synthesize biodegradable plastics, cotton that produces fibers with improved insulating properties, and a variety of crop species with improved protein, fat, vitamin, and mineral composition (6). Products important in human health and medicine such as human milk proteins, insulin, and growth factors may soon be commercially produced by plants containing human genes (6). Many such varieties are currently in the research and development phase (6). However, the transgenic varieties that have been approved for commercial sale to date are focused almost exclusively on increased production and improved plant health (17). The transgenic traits possessed by approved varieties include herbicide tolerance, resistance to insect pests, and resistance to plant diseases (Fig. 2).
|
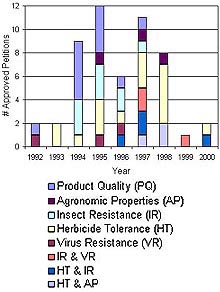
Fig. 2. Applications for deregulation of transgenic crop varieties approved by the USDA, shown by year of application and phenotype category. Data are from the ISB database (17) (click image for larger view). |
|
Transgenic crops have been rapidly adopted by growers, and the global area devoted to transgenic crops increased from 4.3 million acres in 1996 to 69.5 million acres in 1998 (18). In the United States, where most transgenic crops are grown, herbicide resistant soybeans are the transgenic varieties that have been most broadly adopted, and insect resistant corn is ranked second in terms of percentage of acreage planted (Fig. 3). In all, transgenic varieties accounted for 69% of US cotton acreage, 68% of US soybean acreage, and 26% of US corn acreage in 2001 (30). In the mid-1990’s US farmers who adopted transgenic crops such as herbicide-tolerant soybeans and insect-resistant cotton enjoyed significantly increased yields and were able to reduce use of synthetic herbicides and insecticides, respectively (8).
|
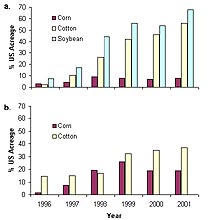
Fig. 3. Percent of all United States production acreage planted with corn, cotton, and soybean varieties that were genetically modified to be (a) herbicide resistant and (b) insect resistant. Varieties with stacked genes (those that are both herbicide and insect resistant) are included under both categories. Data are from the USDA Economic Research Service (41) except for year 2001, which are from National Agricultural Statistics Service (30) (click image for larger view). |
|
Potential environmental risks of transgenic crops
Genetically engineered crops will likely offer many valuable benefits such as increased yields, improved flavor or nutritional quality of foods, and reduced pesticide use. On the other hand, transgenic crops could also pose significant risks for the environment. Two major potential problems for the environment include the creation of new weeds and harmful effects on non-target species. To date, these concerns remain largely theoretical, but this fact should not lull us into a sense of complacency. Genetically modified crops have been planted on a commercial scale for a fairly short time, and it is anticipated that many of the potential problems would only become apparent after many years.
Creation of new weed problems

Fig. 4. Invasive kudzu smothering native shrubs and trees. Photo courtesy of National Park Service, Vicksburg National Military Park (click image for larger view). |
|
Ecologists have repeatedly warned that transgenic crops could lead to the development of new weed problems (40). It may seem counter-intuitive that a plant engineered to benefit humans could end up causing trouble, but many of the world’s most problematic non-native species were actually introduced intentionally for their anticipated benefits (10,21,23,43). Even species that are familiar and beneficial to humans within the plant’s native range can behave in unexpected ways when the plant enters a new environment. For example, kudzu, a benign vine in Japan and China, was widely planted in the southeastern United States as an ornamental, as fodder, and for erosion control (references in 32). Kudzu has since become a pernicious weed that today covers 3 million ha of land and spreads over an additional 50,000 ha each year (Fig. 4; references in 32). In fact, we have very limited ability to identify which species are likely to be successful invaders (22,35,36), let alone to predict the ecological consequences of species introductions.
A common response to concerns about weediness is that, because we are dealing with well-characterized modifications to familiar crop varieties, such surprises are unlikely. First, although it is true that transgenic crops are not entirely unfamiliar, the mingling of genes across phyla and even across kingdoms can result in dramatically altered phenotypes. Second, a common explanation for the success of weeds in new environments is their escape from the natural enemies that limit them in their native habitats. If this argument is correct, then genetic modification of traits such as disease or insect resistance -- traits that confer some “escape from natural enemies” -- could allow populations of crop plants to grow more rapidly, perhaps causing the crop itself to develop into a weedy species that can out-compete native vegetation. Third, genes that enable plants to tolerate herbicide are of particular concern, because plants with these genes could become especially difficult to control. Already, we have witnessed the development of volunteer canola plants in Canada that are resistant to three different herbicides (14). These “super-resistant” canola plants resulted from crosses among three distinct transgenic varieties, each resistant to a single herbicide (14). Concerns about “superweeds” do not seem as far-fetched as they once did now that researchers have documented these “triply resistant” volunteer canola plants. Weed problems may be even more likely for newer genetically modified varieties which often include combinations of transgenic traits such as herbicide resistance plus insect resistance.
Of course, cultivars are usually so domesticated that their overall fitness outside cultivation is low, and the risk of the crops themselves becoming weeds is expected to be small. A more plausible pathway by which transgenic crops could exacerbate weed problems is through the transfer of transgenes to weedy wild relatives. Gene transfer via hybridization with weeds is a common event for many crop species in at least some part of their range. For example, gene flow between crops and wild relatives is very likely for some genetically modified varieties that have been developed such as sunflower in the United States and sugar beet in the United Kingdom (12). In fact, twelve of the world’s thirteen most important crop species (including wheat, rice, corn, and soybean) hybridize with uncultivated species in some part of their distribution (7). In general, regulations governing the safety assessment of transgenic crops require applicants to pay special attention to risks when releases are planned for regions in which hybridization with weeds is possible.
Once hybridization occurs, weed problems would only be exacerbated if the transgenes conferred some fitness benefit to the recipient plant. Whether or not the transgenes will improve plant fitness will depend on the ecological context. For example, resistance to herbicides may entail a metabolic cost, putting individuals containing transgenes at some disadvantage in the absence of the herbicide, but conferring a large fitness advantage in the presence of the herbicide. The few studies that have directly compared the ability of transgenic and non-transgenic crops to establish self-sustaining populations have found either no difference or reduced fitness among transgenic varieties (e.g., 4,5,13). However, because there have often been long time lags between when plants were first released in a novel environment and the time at which the plants developed into pernicious weeds (19,28), the absence of effects in even a decade long study (e.g., 5) should not be considered a guarantee of safety. In addition, models applied to transgenic salmon indicate that transgenes can increase in frequency even though they reduce the overall population growth rate (16). Similar models, tailored to the natural history of crop plants, should be explored.
To estimate how much of an advantage weeds might gain from hybridization with transgenic crops, we can look to studies that have assessed the fitness impacts of reduced herbivore densities. Although such studies seek to understand the importance of herbivores in regulating the abundance of non-transgenic plants, the improved growth and reproduction observed in these studies may reflect the magnitude of effects that would be seen if insect resistance genes were to escape into wild plant populations (27). Although no single herbivore manipulation is terribly informative, meta-analysis can be used to summarize a large collection of such studies across many species and situations to obtain an average measure of herbivore impact (and conversely of the impact of protecting plants against herbivory). Applying this method to results reported in 18 different publications involving 52 different plant-herbivore combinations, the “average effect” of herbivory was very large, with plants protected against invertebrate (primarily insect) herbivores producing more reproductive structures, on average, than 81% of unprotected plants (27). This result is indicative of a likely substantial advantage conferred by any herbivore resistance trait, a magnitude of advantage that could easily translate into enhanced weediness.
On a more optimistic note, it may soon be possible to use molecular techniques to prevent, or at least significantly reduce the likelihood of, transgene spread (11,20). So-called “terminator technology,” linking the transgene of interest to a second gene that renders plants sterile, could be effective at reducing the probability of gene escape (39). However, terminator genes remain controversial because they also prevent seed-saving by farmers, which is an important practice in the developing world. Other genetic means of reducing gene escape that would allow seed-saving are currently under investigation. For example, introducing genes into chloroplast, rather than nuclear, DNA could prevent spread of transgenes by pollen in those species where chloroplasts are exclusively maternally inherited (11). Alternatively, it may be possible to link transgenes tightly with traits that are neutral or beneficial in the crop, but detrimental to weedy relatives (11). A recent development is the possibility of introducing a “recoverable block of function” along with the transgene of interest (20). The focal gene would be tightly linked to a second gene that either kills or sterilizes the plant plus a third gene that allows recovery of function, but only when a chemical or physical trigger is applied to the plant (20). Escaped transgenes would thus render recipient plants inviable.
Until the possibility of gene transfer can be eliminated, risk assessment must consider the likelihood of crosses both among transgenic crop varieties and between transgenic crops and their weedy relatives. Although detailed studies of pollen movement and the performance of hybrids are performed to assess the relative risks for particular transgenic crops (e.g., 5,9), our poor record of predicting the success of introduced species should serve as a warning that a priori detection of all problems is unlikely. Field experiments before the release of transgenic crops are essential, but we must combine these efforts with large-scale monitoring after crops become commercially available.
Effects on Non-target Species
A second and more elusive environmental risk concerns the effects of genetically modified crops on non-target organisms. For example, much concern has been raised over the non-target effects of “Bt crops.” Bt crops are varieties of crops such as corn and cotton that have been genetically modified to express a gene from the bacterium, Bacillus thuringiensis. The Bt gene codes for a protein that is toxic to a fairly specific group of insects. For example, the Bt toxins expressed in Bt corn and in Bt cotton are specific to lepidoptera (butterflies and moths). The major benefits expected from these crops are a reduction in populations of pests, such as the corn earworm (Fig. 5a) and the cotton bollworm (Fig. 5b), and therefore a reduction in pesticide use. Some tests for non-target risks (such as the impacts of Bt toxins on beneficial invertebrates) are routine. Prior to deregulation (approval for commercial planting), Bt crop proteins typically are fed to honeybees and earthworms as a means of assessing non-target effects. These tests have generally reported minimal impacts -- largely because Bt toxins affect highly specific sets of organisms. Despite required tests for non-target effects, surprises have arisen with crops that were approved for deregulation. For example, in 1999 a laboratory study documented that Bt corn pollen dusted on milkweed plants dramatically reduced the survival of monarch butterfly caterpillars (24) (Fig. 6). This and a similar study (15) created tremendous agitation among proponents and opponents of transgenic crops alike. The Environmental Protection Agency (EPA) responded by announcing a “data call-in,” requiring companies that had registered Bt corn to investigate the potential for harm to non-target butterflies. One issue that needed resolution was whether effects detected in lab studies translated into effects in the field under more natural circumstances. For example, adult monarchs might avoid laying eggs on milkweed plants that are coated with corn pollen (34,38). As a result, EPA requested information on the proportion of potential monarch butterfly feeding habitat that occurs in and around Bt corn fields, the lethal concentration of Bt corn pollen for monarch butterfly larvae, how long the lethal concentration of Bt corn pollen remains on milkweed, and whether monarch larvae feed during corn pollen shed.
|

Fig. 5a. Economically important pest species targeted by Bt corn and Bt cotton: the corn earworm (click image for larger view). |
|
|

Fig. 5b. Economically important pest species targeted by Bt corn and Bt cotton: the cotton bollworm (click image for larger view).
|
|

Fig. 6. Monarch butterfly caterpillars feed on a milkweed leaf that has been coated with corn pollen. Photo by Kent Loeffler, Department of Plant Pathology, Cornell University (click image for larger view). |
|
Even after industry’s response to EPA’s data call-in, major questions regarding the effects of Bt corn on monarch butterflies remain unanswered (2). For example, perhaps the relevant baseline is the mortality that monarchs would have suffered from pesticides had the corn not been genetically altered to include Bt transgenes (33). In addition, because pollen from a different variety of Bt corn (notably, one that does not express Bt toxin in pollen at high levels) had no effect on swallowtail butterflies (44), simply shifting production to corn varieties that do not express Bt toxin in their pollen might be sufficient to eliminate non-target effects (33).
Although the Bt and monarch butterfly story is often cited as an example of how risky plant biotechnology could be, it could just as well be cited to show that risk analysis works. Researchers did uncover a non-target impact of Bt pollen on monarchs before major damage had been detected in the field. Other non-target effects are so indirect that it is unlikely they would be routinely examined as part of any research program, much less as part of the regulatory process. For example, adoption of transgenic herbicide-tolerant crops, meant to target weeds, could have consequences for the diversity of birds occurring in and around farmland (42). A mathematical model suggested that the dramatic reductions in weed abundance that are expected as herbicide resistant crops are widely adopted could lead to large declines in the abundance and diversity of bird species (42).
Regardless of whether Bt corn has a serious impact on monarch butterfly populations or whether widespread use of herbicide resistant crops would reduce bird biodiversity, these initial scientific studies make it clear that any blanket guarantees of eco-safety are dishonest. It is likely that we will be confronted by other similar ecological and evolutionary surprises that in hindsight seem so obvious.
Conclusion
Transgenic crops will not single-handedly end world hunger. Neither will transgenic crops become Frankesteinian monsters that take over the world. Careful analysis of the potential risks and purported benefits can help mute the hyperbole of the biotechnology debate. Fortunately, scientific risk assessment is already required or soon to be required in many countries including the United States, the European Union, Canada, Japan, and Australia. Scientists have an important responsibility to contribute to and participate in these risk analyses. Politicians and government agencies can require risk analyses, but only scientists can insure that the studies are credible. In particular, many of the studies that are currently performed in compliance with US regulations are scientifically weak (26,31). Poorly designed tests of safety with few replicates per treatment run a high risk of missing real effects of transgenic crops (26,31). The standards for risk assessment studies must be clarified and elevated, and details of experimental protocols and reasonable sample sizes should be specified.
Even the best scientific risk analyses cannot guarantee that there will be no surprises. For example, it was recently discovered that Bt toxin, which normally degrades quickly, can, under particular circumstances, bind to clay particles in the soil and remain biologically active for at least 230 days (37). As a result of this soil-binding property, Bt toxin may accumulate over time and build up to much higher concentrations than previously anticipated. Findings such as this highlight the point that the effects of genetically engineered crops will be highly variable, making blanket pronouncements of safety untenable.
Finally, scientific risk analysis cannot resolve disputes that involve deeply held values or religious views. However, we should be mindful that risk assessments themselves often subtly entail value judgments. For example, the standard baseline for most comparisons involving plant biotechnology is “current practices”. Thus herbicide-resistant crops are championed because they promote types of herbicide applications (e.g., glyphosate) that are less toxic than other common herbicides. For some environmentalists, this is not the right comparison to make. They feel that public resources should not be spent for research that encourages any usage of herbicide, and that the proper baseline is no-till agriculture, or organic farming, and not current practices. Scientists can best contribute to this debate by clarifying the potential benefits and risks associated with each of these alternative farming practices.
There will always be conflict about technology and its uses. Risk analysis cannot eliminate this conflict, but it can clearly show that portrayals of a technology as “all bad” or “all good” cannot stand up in the light of empirical scrutiny. Ridding ourselves of simplistic, black-and-white views of transgenic crops is an important first step. Beyond that, by helping to design more informative risk analysis procedures, scientists (along with economists, sociologists, ethicists, and others) have the potential to illuminate which technological paths offer the greatest benefit-to-risk ratios. Clearly, we must weigh the benefits that certain transgenic varieties could have for human health (especially in developing nations) against the environmental harm that could result from these same, or other, transgenic varieties. This is not a trivial challenge, particularly because the perceived benefits are often more clearly defined than are the perceived risks. Although risk analysis is often viewed as restrictive to technology, well-designed risk analysis applied to transgenic plants should give us more information about how those cultivars perform under a wide variety of conditions and such knowledge ought to be of as much use to industry as to regulators.
Acknowledgements
Thanks to P. Kareiva and two anonymous reviewers for feedback on an earlier draft of this manuscript.
Additional Resources
USDA APHIS, Agricultural Biotechnology
US EPA, Office of Pesticide Programs, Biopesticides
The Royal Society of Canada, Expert Panel on the Future of Food Biotechnology
National Research Council Report on Genetically Modified Pest-Protected Plants
Congressional Research Service, Report for Congress, Food Biotechnology in the United States: Science, Regulation, and Issues
Literature Cited
1. Angus Reid Group Inc. 2000. Awareness of genetically modified foods wide but knowledge inch deep. Online. Angus Reid Media Release Center, June 8.
2. Brower, L. 2001. Canary in the cornfield: The Monarch and the Bt controversy. Online. Orion, Spring 2001.
3. Carbone, J. and McLean, M. R. 2001. Genetically modified foods: The creation of trust and access to global markets. Business and Professional Ethics Journal. In press.
4. Crawley, M. J., Hails, R. S., Rees, M., Kohn, D., and Buxton, J. 1993. Ecology of transgenic oilseed rape in natural habitats. Nature 363:620-623.
5. Crawley, M. J., Brown, S. L., Hails, R. S., Kohn, D. D., and Rees, M. 2001. Transgenic crops in natural habitats. Nature 409:682-683.
6. Dunwell, J. M. 1999. Transgenic crops: The next generation, or an example of 2020 vision. Ann. Bot. 84:269-277.
7. Ellstrand, N. C., Prentice, H. C., and Hancock, J. F. 1999. Gene flow and introgression from domestic plants into their wild relatives. Ann. Rev. Ecol. Syst. 30:539-563.
8. Fernandez-Cornejo, J. and W. D. McBride. 2000. Genetically engineered crops for pest management in U.S. agriculture: Farm-level effects. Online. USDA, Economic Research Service, Resource Economics Division, Agricultural Economic Report No. 786.
9. Giddings, G. 2000. Modeling the spread of pollen from Lolium perenne. The implications for the release of wind-pollinated transgenics. Theor. Appl. Genet. 1000:971-974.
10. Gordon D. R. and Thomas, K. P. 1997. Florida's invasion by nonindigenous plants: History, screening, and regulation. Pages 21-37 in: Strangers in paradise: Impact and management of nonindigenous species in Florida. Simberloff, D., Schmitz, D. C., and Brown, T. C., eds. Island Press, Washington D.C.
11. Gressel, J. 1999. Tandem constructs: Preventing the rise of superweeds. Tibtech 17:361-366.
12. Hails, R. S. 2000. Genetically modified plants - the debate continues. Trends Ecol. Evol. 15:14-18.
13. Hails, R. S., Rees, M., Kohn, D. D., and Crawley, M. J. 1997. Burial and seed survival in Brassica napus subsp. oleifera and Sinapis arvensis including a comparison of transgenic and non-transgenic lines of the crop. Proc. R. Soc. Lond. B 264:1-7.
14. Hall, L., Topinka, K., Huffman, J., Davis, L., and Good, A. 2000. Pollen flow between herbicide-resistant Brassica napus is the cause of multiple-resistant B. napus volunteers. Weed Science 48:688-694.
15. Hansen-Jesse, L. C. and Obrycki. J. J. 2000. Field deposition of Bt transgenic corn pollen: Lethal effects on the monarch butterfly. Oecologia 125:241-248.
16. Hedrick, P. W. 2001. Invasion of transgenes from salmon or other genetically modified organisms into natural populations. Can. J. Fish. Aquat. Sci. 58:841-844.
17. Information Systems for Biotechnology. 2001. Crops no longer regulated by USDA. Online database. Virginia Polytechnic Institute and State University, ISB.
18. James, C. 2000. Global status of transgenic crops: Challenges and opportunities. Pages 1-7 in: Plant Genetic Engineering: Towards the Third Millennium. Arencibia, A. D., ed. New York: Elsevier.
19. Kowarik, I. 1995. Time lags in biological invasions with regard to the success and failure of alien species. Pages 15-38 in: Plant invasions: General aspects and special problems. Pysek, P., Prach, K., Rejmanek, M., and Wade, M., eds. SPB Academic Publishing, Amsterdam.
20. Kuvshinov, V., Koivu, K., Kanerva, A., and Pehu, E. 2001. Molecular control of transgene escape from genetically modified plants. Plant Science 160:517-522.
21. Le Floc'h E., Le Houerou, H. N., and Mathez, J. 1990. History and patterns of plant invasion in Northern Africa. Pages 105-133 in: Biological invasions in Europe and the Mediterranean basin. di Castri, F., Hansen, A. J., and Debussche, M., eds. Kluwer Academic Publ., Dordrecht.
22. Lodge, D. M. 1993. Biological invasions: Lessons for ecology. Trends Ecol Evol 8:133-137.
23. Lonsdale, W. M. 1994. Inviting trouble: Introduced pasture species in northern Australia. Austral. J. Ecol. 19:345-354.
24. Losey, J. E., Rayor, L. S. and Carter, M. E. 1999. Transgenic pollen harms monarch larvae. Nature 399:214.
25. Lucca, P., Hurrell, R., and Potrykus, I. 2001. Genetic engineering to improve the bioavailability and the level of iron in rice grains. Theor. Appl. Gen. 102:392-397.
26. Marvier, M. 2001. Ecology of transgenic crops. American Scientist 89:160-167.
27. Marvier, M. A. and Kareiva, P. 1999. Extrapolating from field experiments that remove herbivores to population-level effects of herbivore resistance transgenes. Pages 57-64 in: Proceedings of a Workshop on: Ecological Effects of Pest Resistance Genes in Managed Ecosystems. Traynor, P. L. and Westwood, J. H., eds. Information Systems for Biotechnology, Blacksburg, VA.
28. Marvier, M. A., Meir, E., and Kareiva, P. M. 1999. How do the design of monitoring and control strategies affect the chance of detecting and containing transgenic weeds? Pages 109-122 in: Methods for Risk Assessment of Transgenic Plants, Vol. 3. K. Ammann, Y. Jacot, V. Simonsen, and G. Kjellsson, eds. Birkhauser Verlag Press, Basel.
29. Nash, M. J. 2000. Grains of hope. Time Magazine 156:38-46.
30. National Agricultural Statistics Service. 2001. Acreage. Online. Agricultural Statistics Board, USDA, Washington, D.C.
31. National Research Council. 2001. Ecological Monitoring of Genetically Modified Crops: A Workshop Summary. National Academy Press, Washington, D.C.
32. Pappert, R. A., Hamrick, J. L., and Donovan, L. A. 2000. Genetic variation in Pueraria lobata (Fabaceae), an introduced, clonal, invasive plant of the southeastern United States. Am. J. Bot. 87:1240-1245.
33. Pimentel, D. S. and Raven, P. H. 2000. Bt corn pollen impacts on nontarget Lepidoptera: Assessment of effects in nature. Proc. Nat. Acad. Sci. 97:8198-8199.
34. Poppy, G. 2000. GM crops: Environmental risks and non-target effects. Trends Plant Sci. 5:4-6.
35. Pysek, P., Prach, K., and Smilauer, P. 1995. Relating invasion success to plant traits: An analysis of the Czech alien flora. Pages 39-60 in: Plant invasions: General aspects and special problems. Pysek, P., Prach, K., Rejmánek, M., and Wade, M., eds. SPB Academic Publishing, Amsterdam.
36. Roy, J. 1990. In search of the characteristics of plant invaders. Pages 335-352 in: Biological invasions in Europe and the Mediterranean basin. di Castri, F., Hansen, A. J., and Debussche, M., eds. Kluwer Academic Publ., Dordrecht.
37. Saxena, D., Flores, S., and Stotzky, G. 1999. Insecticidal toxin in root exudates from Bt corn. Nature 402:480.
38. Schuler, T. H., Potting, R. P. J., Denholm, I., and Poppy, G. M. 1999. Parasitoid behavior and Bt plants. Nature 400:825-826.
39. Service, R. F. 1998. Seed-sterilizing ‘terminator technology’ sows discord. Science 282:850-851.
40. Tiedje, J. M., Colwell, R. K., Grossman, Y. L., Hodson, R. E., Lenski, R. E., Mack, R. N., and Regal, P. J. 1989. The planned introduction of genetically engineered organisms: Ecological considerations and recommendations. Ecology 70:298-315.
41. USDA Economic Research Service. 2001. Agricultural biotechnology: Adoption of biotechnology and its production impacts. Online. Agricultural Biotechnology Briefing Room.
42. Watkinson, A. R., Freckleton, R. P., Robinson, R. A., and Sutherland, W. J. 2000. Predictions of biodiversity response to genetically modified herbicide-tolerant crops. Science 289:1554-1557.
43. Williams, M. C. 1980. Purposefully introduced plants that have become noxious or poisonous weeds. Weed Science 28:300-305.
44. Wraight, C. L., Zangerl, A. R., Carroll, M. J., and Berenbaum, M. R. 2000. Absence of toxicity of Bacillus thuringiensis pollen to black swallowtails under field conditions. Proc. Nat. Acad. Sci. 97:7700-7703.
45. Ye, X., Al-Babili, S., Kloti, A., Zhang, J., Lucca, P., Beyer, P., and Potrykus, I. 2000. Engineering the provitamin A (β-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. Science 287:303-305.
