|
Prepared by
Laurene Levy, USDA-APHIS, PPQ, Beltsville, MD laurene.e.levy@usda.gov

Vern Damsteegt, USDA-ARS, FDWSRU, Ft. Detrick, MD damsteeg@ncifcrf.gov

Ralph Scorza, USDA-ARS, AFRS, Kearneysville, WV rscorza@afrs.ars.usda.gov

Maria Kölber, PHSCS, Budapest, Hungary novved@elender.hu
Levy, L., Damsteegt, V., Scorza, R. and Kolber, M. 2000. Plum Pox Potyvirus Disease of Stone Fruits. APSnet Features. Online. doi: 10.1094/APSnetFeature-2000-0300 |

|
Abstract
Plum pox potyvirus, the cause of the most destructive and most feared viral disease of Prunus, (plum pox or Sharka) has been established in North America. Following recognition of symptomatic peach fruit and the positive confirmation of the causal agent of the disease in October, 1999, an official announcement of the presence of the dreaded disease in Adams County, Pennsylvania was made jointly by the Animal & Plant Health Inspection Service and the Pennsylvania Department of Agriculture. Plum pox strains are capable of causing disease in peaches, plums, apricots, nectarines, almonds, sweet and sour cherries, as well as in other selected Prunus and non-Prunus species. Aphid-transmitted by more than 20 different aphid species in a stylet-borne manner, the virus also is spread by movement of nursery stock, budwood, and grafting. Control measures are discussed including development of a highly resistant transgenic plum line.
Introduction:
|

Figure 1. Click Image for enlargement and more information.
|
Plum pox symptoms were first observed in plums by plum growers in Bulgaria between 1915 and 1918, at the close of World War I, although some reports indicate symptoms were seen in Macedonia as early as 1910. However the first paper describing the viral nature of the disease did not appear until 1932 when Atanosoff named it "Sarka po slivite" meaning "Pox of Plum" (=Sharka). Christoff (1934) observed Sharka affecting apricots in Bulgaria in 1933, but it was not until the early 1960’s that it was reported affecting peaches in Hungary (Figure 1).Between 1932 and 1960 the disease moved north and east from Bulgaria into Yugoslavia, Hungary, Romania, Albania, Czechoslovakia, Germany and Russia. The disease was observed mainly in plums and apricots until the 1960’s and was never observed in peaches in Bulgaria, or Yugoslavia until the 1980’s and only then in peaches which had come from Hungary. In addition, the strain was different from that found in plums.
Following World War II Sharka progressed into western Europe reaching Germany and Austria by the late 1950’s. By the mid-60's, Sharka had reached The Netherlands, Switzerland, Greece, England and Turkey. France, Italy, and Belgium by the early 70’s; Spain and Portugal by the early 80’s; Egypt, Syria, and Cyprus by the late 80’s; Chile in 1992; India in 1994; and the USA in 1999.
|
Table 1. Plum Pox Status and Associated Geographic Distribution
|
|
Disease Status |
Country |
|
Restricted Distribution |
Albania, Austria, Cyprus, Czech Republic, France, Italy, Luxembourg, Moldova, Norway, Portugal, Southern Russia, Slovenia, Spain, Syria, Turkey, Ukraine, United Kingdom, United States. |
|
Widespread |
Bulgaria, Croatia, Germany, Greece, Hungary, Poland, Romania, Slovakia, Former Yugoslavia |
|
Introduced, Established |
Azores, Bosnia-Herzegovina, Egypt, Former USSR, India, Lithuania |
|
Introduced, Presumably Eradicated |
Belgium, Netherlands, Switzerland |
|
Present Status Unknown |
Chile, Denmark |
| Please Note: An earlier version of this table listed plum pox as being present in Australia. This was an error. Plum pox has never been detected in Australia. |
The relentless progress of the disease in Europe and the severity of the disease led to the development of the Sharka International Working Group in the 1970’s within the framework of the European Plant Protection Organization (EPPO), which allowed coordination of research and a free flow of information between countries. Quarantine regulations were imposed between countries exchanging Prunus germplasm, which slowed the movement. Despite this effort, PPV has been moving and changing. Plum pox virus belongs to the genus Potyvirus in the family Potyviridae. Members of the genus Potyvirus have virions which are flexuous filaments with no envelopes, are aphid-transmitted in a non-persistent, stylet-borne manner, mechanically transmitted, and may or may not be seed-transmitted.
|

Figure 2.
Click for enlargement and more information.
|
|
PPV has a single molecule of positive sense, ssRNA, that is 9.7 kb; virions are approximately 764 X 20 nm. The genome is expressed as a 350 kDa polyprotein precursor that is proteolytically processed by viral and host proteases into seven smaller functional proteins including a 3’ coat protein and a helper component. It is the only recognized potyvirus infecting Prunus. The introduction of PPV to a new country or region is usually through propagative materials and the subsequent distribution of contaminated materials. The secondary spread can be rapid and results from aphid transmission (Figure 2).
Plum pox virus has been transmitted by at least 20 aphid species, although only 4-6 are considered important vectors (Table 2). The efficiency of transmission is dependent on the virus strain, host cultivars, age of the host cultivars, aphid species, and time of year. The most important aphid vectors reported from several countries are Brachycaudus cardui, B. helichrysi, Myzus persicae, and Phorodon humuli. Reports vary from country to country, however, natural virus spread is low in July and August but high in spring and autumn. Spring flights of B. helichrysi, M. persicae, and P. humuli are most important for spread within and between orchards. Analysis of spacial distribution of PPV by Gottwald et al. (1995) suggest a lack of movement by aphid vectors to immediately adjacent trees and a preference for movement several tree spaces away.
Aphids can acquire the virus in probes as short as 30 seconds, and can transmit for up to 1 hour. Aphids that have been starved before feeding can transmit for up to 3 hours after acquisition. There is no correlation between the ability to transmit PPV and the ability to colonize Prunus. PPV can be spread in orchards by transient aphids as efficiently as aphids colonizing Prunus (Labonne et al., 1995).
Aphids were found to transmit PPV within 100-120 m of the source plants, but they have been shown to carry the virus on their stylets for several kilometers if starved during flight.
|
Table 2. Aphid species shown to be vectors of plum pox virus. |
|
Aphid Species |
Colonizes Prunus |
Host |
|
Aphis arbuti |
No |
Arbutus unedo |
|
A. craccivora* |
No |
Polyphagous |
|
A. fabae |
N | o |
Polyphago |
us |
|
A. gossypii* |
No |
Polyphagous |
|
A. hederae |
No |
Hedera helix |
|
A. spiraecola* |
Occasionally |
Polyphagus; Apple; Citrus |
|
Brachycaudus cardui |
Yes |
Prunus; Compositae |
|
B. helichrysi** |
Yes |
Prunus; Compositae |
|
B. persicae* |
Yes |
Prunus |
|
Dysaphis plantaginea |
No |
Apple; Plantago |
|
D. pyri |
No |
Pear; Gallium |
|
Hyalopterus pruni* |
Yes |
Prunus; Fragmites |
|
Macrosiphum rosae |
No |
Rosa; Dipsaceae |
|
Megoura rosae |
No |
Leguminoseae |
|
Myzus persicae** |
Yes |
Polyphagous |
|
M. varians |
Yes |
Peach; Clematis |
|
Phorodon humuli** |
Yes |
Prunus; Hop |
|
Rhopalosiphum padi |
No |
Prunus padus; Gramineae |
|
Sitobion fragariae |
No |
Rosa; Gramineae |
|
Ureleucon sonchi |
No |
Lactuca; Sonchus |
| *Recognized aphid vectors, ** Most important vectors. Data communicated personally by J. B. Quiot, INRA, Montpellier, France. |
Plum pox virus has a broad experimental host range although it has a rather restricted natural host range within the genus Prunus. It infects peaches, plums, apricots, nectarines, almonds, and sweet and tart cherry. Virus isolates vary in their reaction to different hosts, and not all strains or isolates infect the same host range. Prunus species that have been proven to be hosts in nature, or by inoculation trials followed by back transmissions include:
- Prunus armeniaca - Apricot
-
P. persica - Peach
-
P. persica var. nectarina - Nectarine
-
P. domestica - Garden plum (prune)
-
P. salicina - Japanese plum
-
P. insititia - Damson plum
-
-
P. cerasifera - Myrobalan plum
-
P. glandulosa - Dwarf flowering almond, Cherry almond
-
P. avium - Sweet cherry
-
P. cerasus - Sour (tart) cherry
-
P. amygdalus - Almond
Wild Prunus may serve as an important secondary host of PPV and can have an impact on plum pox epidemiology and control (Polak, 1997). In addition to the above natural hosts, several wild Prunus species are susceptible:
- P. spinosa - Blackthorn
- P. americana - American plum
- P. bessey - Western sand cherry
- P. mahaleb - Mahaleb or St. Lucie cherry
- P. mume - Japanese apricot
- P. pumila - Sand cherry
- P. hortulana - Hortulan plum
- P. davidana - David peach, Chinese wild peach
- P. tomentosa - Nanking cherry
- P. nigra - Canada plum
- P. maritime - Beach plum
- P. laurocerasus - English cherry-laurel
Many non-Prunus species, in at least nine plant families, have been infected artificially with one or more strains of the plum pox virus, and in some cases found naturally infected in the field. Most of these are herbaceous annuals but a few are perennial or woody and could serve as over-wintering sources of the virus. Some of the common hosts include:
- Campanula rapunculoides
- Chenopodium quinoa
- C. species
- Lamium album
- L. amplexicaule
- L. purpureum
- Lupinus albus
- Lycium barbarum
- L. halimifolium
- Medicago lupulina
- Melilotus officinalis
- Ranunculus acer
- R. arvensis
- R. repens
- Silene vulgaris
- Solanum dulcamara
- Trifolium incarnatum
- T. pratense
- T. repens
- Zinnia elegans
- Z. violacea
In addition the following are the more important herbaceous indicator or propagation hosts of plum pox virus:
-
Chenopodium foetidum
-
Nicotiana benthamina
-
N. bigelowii
-
N. clevelandii
-
N. occidentalis #37 B
-
N. edwardsonii
-
N. megalosiphon
-
N. tabacum
-
N. physalodes
-
Pisum sativum cv. Colmo
In Prunus, plum pox virus symptoms appear on leaves, fruits, flowers, and seeds. The severity of the symptoms varies according to the Prunus species and cultivar, PPV strain, season and location. Leaves and fruit show chlorotic (yellowing) and necrotic (browning) ring patterns, and chlorotic bands or blotches (Figure 3). Leaves and fruit also can be absent of symptoms, or have symptoms that are ameliorated during the growing season. The fruit of apricot and plum can be misshapen and deformed (Figure 4), or rings may be present on their stones (Figure 5). Some peach cultivars may show color-breaking symptoms on the flower petals (Figure 6). Virus infection can cause considerable losses. About 100 million stone fruit trees in Europe are currently infected, and susceptible cultivars can result in 80-100% yield losses (Kegler, 1998). In eastern and central Europe, sensitive plum varieties can exhibit premature fruit drop and bark splitting (Figure 7). Some sweet cherry fruits develop chlorotic and necrotic rings, notched marks, and premature fruit drop (Nemchinov et al., 1998).
|

Figure 3
|
|

Figure 4 |
|

Figure 5
|

Figure 6
|

Figure 7
| |
|
Click on any image for enlargement and more information. |
Similar to other plant viruses, plum pox is comprised of several strains based on biology, serological reactions, and molecular and biological data. To date, four strains or serogroups have been characterized that are referred to as PPV strain M, D, EA, and C (Kerlan and Dunez, 1979; Wetzel et al., 1991a; Kalashyan et al., 1994; Crescenzi et al., 1994). Individual isolates within each strain/serogroup may vary biologically. PPV-D is the Dideron strain that was originally isolated from apricot in southeastern France, and is the most common strain of the virus in western Europe. PPV-D also occurs in the Western hemisphere in Chile, and now recently in the US (Pennsylvania). Apricot, peach, and plum are the natural Prunus hosts of the D strain. This strain is known not to be seed-transmitted, can be difficult to transmit to experimental hosts, is less efficiently aphid-vectored, and is the non-epidemic form of plum pox. PPV-M is the Marcus strain that was originally isolated from peach in northern Greece, and is the most common strain of the virus in southern, eastern, and central Europe. Peach is the main natural Prunus host, however, apricot and plum are susceptible. PPV-M has been reported to be seed-transmitted in some cultivars in eastern and central Europe (Nemeth and Kolber, 1983), is transmitted to experimental hosts easily, is spread rapidly by aphids and is considered to be the epidemic form of the virus. One isolate of PPV-M from France is very aggressive and produces necrosis in peach leaves causing leaf drop and dieback (Candresse et al. 1993). PPV-EA is the El Amar strain that was originally isolated from apricot in Egypt. So far, PPV-EA is only found in this North African region. Although little information is available for the EA strain, some characteristics are similar to the M strain. PPV-C is the cherry strain that was originally isolated from tart (sour) cherry from Moldova. The natural Prunus host range of PPV-C is both sweet and tart cherry. The cherry strain can be experimentally transmitted to other Prunus species. PPV-C is transmitted efficiently by aphids, and has a wider experimental host range than other PPV strains. This strain is present in eastern and central Europe, and Italy.
The properties that separate plum pox virus strains can be exploited for detection purposes. The simplest method for detection of plum pox is using biological index hosts. PPV can be detected in herbaceous indicator hosts by mechanical inoculation to such diagnostic hosts as Chenopodium foetidum and several Nicotiana species. The virus is also detected reliably in woody indicator plants by chip budding to hosts such as GF 305 and GF 31 peach, and Prunus tomentosa (IR473 X IR474 hybrid). The latter has the ability to differentiate the M and D strains based on symptoms (Damsteegt et al., 1997) (Figure 8). The biochemical properties of the virus also have been utilized for detection purposes. PPV strains can be differentiated in western blots according to the molecular weight of the viral coat protein. M strains have a coat protein mass of 38 kDa, the D strains are 36 kDa in size, and some strains are intermediate in size (Ravelonandro, 1998).
 |
 |
| Figure 8 |
Figure 9 |
| Click on either image for enlargement and more information. |
The detection of plum pox virus has followed closely the advances in the field of diagnostic plant pathology, and in some instances has helped to advance the field. Plum pox virus detection by ELISA was included in some of the first work done by Clark and Adams on the application of this technology for the detection of plant viruses (Clark and Adams, 1977; Adams, 1978). Similar to other tree fruit viruses, plum pox virus concentration can be low at certain times of the year and in certain Prunus cultivars (Figure 9). The virus is unevenly distributed in trees that are newly infected or have some degree of resistance, however, once an infection is established it can reach high titers in plant tissues such as leaves, flowers, and fruit in the spring and early summer. Several polyclonal and monoclonal antibodies have been developed and are used worldwide for PPV detection. Recently, monoclonal antibodies have been developed for the universal detection of all PPV strains (5B-IVIA) (Cambra et al., 1994) and for the four serogroups, M, D, C, and EA strains (Crecenzi et al., 1998; Boscia et al, 1997; Myrta et al, 1998).
More sensitive and accurate detection of plum pox became possible in the 1980’s through the application of cDNA and cRNA probes, which helped to overcome the problem of low concentration of the virus (Varveri et al., 1987, 1988). As plant pathologists applied polymerase chain reaction technology to plant virus detection in the early 1990’s, plum pox virus was among the first viral targets amplified (Wetzel et al.,1991b). PCR tests have been developed to universally or specifically amplify PPV strains based on characteristics of the potyvirus genome, such as the variable N-terminal region of the viral coat protein and the conserved 3’ non-coding (fingerprint) region (Figure 10) (Levy and Hadidi, 1994; Candresse et al, 1998). 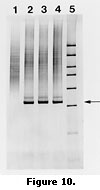 Differentiation of the four strains is possible using PCR-RFLP by digestion of the coat protein and replicase amplification products (Candresse et al., 1994; Hammond et al., 1998). Low concentration of the virus occurs during the summer, and in some tissues such as bark. The application of PCR increased the sensitivity and accuracy of detection over ELISA and hybridization, but the development of immunocapture PCR (IC-PCR) increased the sensitivity of PCR to about 5000 times that of ELISA. The technique developed by Wetzel for PPV detection used antibody trapping of virus particles prior to amplification (Wetzel et al., 1992). This technique also overcame the problem associated with carryover inhibitors of PCR from plant sap. Another technique was developed for simplification of PCR detection of PPV called print capture PCR (PC-PCR) (Olmos et al., 1996). Plant tissue or insects are pressed onto filter paper that is dropped into the PCR tube for amplification. The applicability of the above diagnostic techniques were compared by Lopez et al. (1999) and is presented in Table 3.
Differentiation of the four strains is possible using PCR-RFLP by digestion of the coat protein and replicase amplification products (Candresse et al., 1994; Hammond et al., 1998). Low concentration of the virus occurs during the summer, and in some tissues such as bark. The application of PCR increased the sensitivity and accuracy of detection over ELISA and hybridization, but the development of immunocapture PCR (IC-PCR) increased the sensitivity of PCR to about 5000 times that of ELISA. The technique developed by Wetzel for PPV detection used antibody trapping of virus particles prior to amplification (Wetzel et al., 1992). This technique also overcame the problem associated with carryover inhibitors of PCR from plant sap. Another technique was developed for simplification of PCR detection of PPV called print capture PCR (PC-PCR) (Olmos et al., 1996). Plant tissue or insects are pressed onto filter paper that is dropped into the PCR tube for amplification. The applicability of the above diagnostic techniques were compared by Lopez et al. (1999) and is presented in Table 3.

Table 3. PPV Detection Techniques Compared (Data interpreted from J.J. Lopez, et al., Journal of Biotechnology, 1999)
 |
|
Assaya |
Diffi-
culty |
Speed |
Possibility for Routine Testing |
Reliable in Winterb |
Samples/
Day |
Cost/
Sample In Eurosc |
|
Biological Indexing |
5d |
1e |
3f |
5 |
450 |
45-90 |
|
ELISA |
4 |
5 |
5 |
1 |
300 |
0.7 |
|
Hybridizationg |
3 |
5 |
4 |
1 |
300 |
1.1 |
|
RT-PCRh |
2 |
4 |
1 |
1 |
100 |
1.3 |
|
IC-RT-PCRi |
2 |
4 |
1 |
2 |
120 |
1.4 |
|
PCR-ELISAj |
1 |
3 |
1 |
2 |
100 |
2.6 |
aAssay choice listed from least sensitive (ELISA) to most sensitive (nested PCR-ELISA). Biological indicators are the most sensitive assay.
bAll techniques are equally sensitive in spring tests. Scale range is from most reliable (5) to least reliable (1).
c1 Euro equals $0.9605 US dollars on 2/2/00.
d-f Methods rate from acceptable (1) to optimum (5).
gHybridization using cDNA or cRNA probes in southern or dot blots.
h-jRT-PCR is reverse transcriptase-polymerase chain reaction. IC-RT-PCR is immunocapture RT-PCR where virus particles are first trapped by antibodies prior to RT-PCR. PCR-ELISA is PCR that is followed by capture and hybridization in microtiter plates to labeled probes and detection similar to ELISA. |
Detection of PPV is only part of preventing the virus spread to a new area, region, or country. Plum pox prevention includes the following:
- Regulations regarding the importation and movement of propagative materials and commercial propagants.
- Production of virus-free trees through the indexing of mother trees and the selection of virus-free budwood and rootstocks.
- Indexing of germplasm in quarantine (indexing and therapy of infected precious material).
- Production and use of resistant cultivars.
- Annual visual inspections and surveys in orchards and nurseries.
Unlike fungal or bacterial plant pathogens that can be controlled chemically, antiviral treatments to prevent or control PPV in the field are not available. The most effective means of control are the following:
- Early detection using surveys and subsequent removal and destruction of infected trees (Figure 11).
- Intercropping with resistant Prunus cultivars, and the use of non-host biological barriers (tree buffers).
- Chemical control of migratory or over-wintering aphids.
- Use of resistant cultivars and rootstocks.
- Development of resistant cultivars through genetic engineering and/or conventional breeding programs.
|

Figure 11
Click image for enlargement
and more information.
|
Despite the fact that resistance to this disease has been sought ever since its discovery, there are few reliable reports of high-level resistance in Prunus. There are many conflicting reports of resistance and these inconsistencies result, in part, from the many definitions of the broad term "resistance". These definitions include immunity, where the plant cannot be infected; resistance, where disease is localized to the infection site (the degree of localization is the quantitative aspect of resistance and can vary widely); hypersensitivity, a highly susceptible reaction that provides resistance if localized; and tolerance, where a plant is fully infected but expresses few, if any, symptoms (Hartmann, 1998; Kegler et al., 1998). Many reports of resistance to PPV have not been particularly specific in defining the term "resistance". Furthermore, resistance ratings can be difficult to interpret and to compare between reports because infection may be affected by factors including the PPV strain or isolate used as inoculum, the method of inoculation, the time from inoculation to rating, the part of the plant rated (e.g. fruit or leaves), and the sensitivity of the method of detecting infection (visual symptoms, ELISA, PCR, etc.). These factors have caused some cultivars to be rated alternately as immune and susceptible, or as resistant and tolerant (Kegler et al., 1998). A critical reading of the literature on resistance to PPV suggests that there is not a source of high-level resistance or immunity to PPV in Prunus that will protect trees against all strains of the virus. There are sources of resistance, generally multigenic, that will provide moderate levels of resistance or tolerance to at least some strains of the virus (Kegler et al., 1998). While moderate levels of resistance or tolerance allow growers to produce and market fruit, high levels of resistance to PPV are needed to prevent continual spread by aphids.
Ideally, new PPV resistant varieties would be highly resistant to multiple strains of the virus. The virus should not be able to replicate in the resistant variety or replication would be at such low levels that the virus could not cause symptoms nor be transmitted from that variety by aphids.
Developing PPV-resistant stone fruits through conventional approaches has been utilized exclusively to date. This approach has met with limited success due to the mulitgenic nature of PPV resistance identified thus far, and the strain-specific nature of this resistance. With generation cycles ranging from 3 to 6 years for Prunus species (Sherman and Lyrene, 1983), the time necessary to incorporate a high level of multigenic resistance to many PPV strains plus incorporate high levels of fruit quality, yield potential, cold-tolerance, and resistance to other diseases can be greater than the lifetime of a breeder. Progress is painstakingly slow. Institutions and individuals must be willing to support these long-term programs. The development of molecular markers for PPV resistance would speed the breeding process, but marker development for tree fruits is not a trivial task and requires substantial time, labor and expense. One of the most important aspects of developing markers for PPV resistance would be to evaluate critically the resistance of parental material in terms of the degree and breadth (over strains) of resistance. Another is to evaluate the resistance of progeny in segregating, mapping populations using artificial inoculations over several years with sensitive detection methodologies.
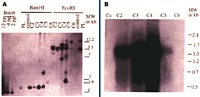
An alternative to the traditional methods of variety development and one that provides new gene resources for resistance breeding is the transformation of plants with viral genes such as those for coat protein (CP). Transgenic plants expressing viral genes have been shown to exhibit varying degrees of resistance to the homologous virus or to viruses closely related to the source of the transgene (Beachy et al., 1990). The PPV-CP gene has been transferred into plum (P. domestica) (Scorza et al., 1994). We have shown that one transgenic plum line, C5, is highly resistant to PPV and has remained so for over 5 years in greenhouse tests using chip bud and aphid inoculation with both the D and M strains of PPV (Ravelonandro et al., 1997, 1998). Field tests in Europe have confirmed that C5 is highly resistant to PPV (highly resistant as defined above) (Malinowski et al., 1998) (Figure 12). This transgenic line contains multiple copies of the PPV-CP transgene, produces low levels of PPV-CP RNA, and no detectable PPV-CP (Scorza et al., 1994) (Figure 13). Resistance appears to be due to post-transcriptional gene silencing (Scorza et al., in preparation). The multicopy block of genes is inherited as a single dominant gene and progeny carrying this transgene insert are resistant to PPV (Scorza et al., 1998).
 |
 |
| Figure 12 |
Figure 13 |
Click either image for enlargement and more information. |
Transformation could be used to transfer PPV resistance to widely grown cultivars, although to date transformation of most stone fruit cultivars has not been possible. Alternatively, resistant transgenic lines, such as C5, developed from seedling transformation could be used as resistant parents in breeding programs. The advantage of a clone like C5 is that the inheritance as a single dominant gene for resistance allows for rapid selection in segregating populations. Using PCR to detect the gene, seedlings without the transgene could be eliminated before field-planting. Only trees carrying the transgene would be grown in the field and only those with desirable agronomic traits would undergo inoculation trials.
Unlike most herbaceous crops, fruit trees are vegetatively propagated, and remain in the field for many years. Some cultivars have been grown for literally hundreds of years. Therefore, the durability of resistance based on a single gene is questionable. New sources of resistance genes, from within and outside the Prunus genome must be exploited. These genes should be combined with existing genes through transformation and hybridization to provide broad-based, horizontal resistance.
In order to develop efficient, progressive programs for developing PPV-resistant Prunus, it will be necessary to critically review the PPV-resistance literature, forge strong collaborations between breeders, virologists, pathologists, horticulturists, entomologists, and molecular biologists world-wide. It is also important to critically evaluate germplasm; and to utilize conventional hybridization and selection, molecular mapping, and gene transfer technologies for germplasm and cultivar development.
DOWNLOAD A PLUM POX FACT SHEET IN FULL COLOR
Literature Cited
1. Adams, A.N. (1978). The detection of plum pox virus in Prunus species in enzyme-linked immunosorbent assay (ELISA). Annals of Applied Biology, 90:215-221.
2. Atanassov, D. (1932). Plum pox. A new virus disease. Ann. Univ. Sofia, Fac. Agric. Silvic. 11, 49-69.
3. Beachy, R.N., Loesch-Fries, S., Tumer, N.E. (1990). Coat protein-mediated resistance against virus infection. Ann. Rev. Phytopath. 28, 451-474.
4. Boscia, D., Zeramdini, H., Cambra, M., Potere, O., Gorris, M.T., Myrta, A., DiTerlizzi, B., and V. Savino. (1997). Production and characterization of a monoclonal antibody specific to the M serotype of plum pox potyvirus. European Journal of Plant Pathology, 102:477-480.
5. CABI/EPPO, (1998). Plum pox potyvirus. Distribution Maps of Quarantine Pests for Europe No. 320. Wallingford, UK, CAB International.
6. Cambra, M., Asensio, M., Gorris, M.T., Perez, E., Camarasa, E., Garcia, J.A., Moya, J.J., Lopez-Abella, D., Vela, C., and A. Sanz. (1994). Detection of plum pox potyvirus using monoclonal antibodies to structural and non-structural proteins. Bulletin OEPP/EPPO, 24:569-577.
7. Candresse, T., Cambra, M., Dallot, S., Lanneau, M., Asensio, M., Gorris, M.T., Revers, F., Macquaire, G., Olmos, A., Boscia, D., Quiot, J.B., and J. Dunez. (1998). Comparison of monoclonal antibodies and polymerase chain reaction assay for the typing of isolates belonging to the D and M serotypes of plum pox potyvirus. Phytopathology 88:198-204.
8. Christoff, A. (1934). Mosaikkrankheit oder Viruschlorose, bei Apfeln. Eine neue Viruskrankheit. Phytopath. Z. 7:521-536.
9. Clark, M.F. and A.N. Adams. (1977). Characteristics of a microplate method of enzyme-linked immunosorbent assay for the detection of plant viruses. J. Gen. Virol. 34:475-483.
10. Crescenzi, A., Nuzzaci, M., Levy, L., Piazzolla, P., and A. Hadidi. (1994). Infezioni di sharka su ciliegio dolce in Italia meridionale. Informatore Agrario, 34:73-75.
11. Damsteegt, V.D., Waterworth, H.E., Mink, G.I., Howell, W.E., and L. Levy. (1997). Prunus tomentosa as a diagnostic host for the detection of plum pox virus and other Prunus viruses. Plant Dis. 81:329-332.
12. EPPO, (1999). EPPO PQR database (Version 3.8). Paris, France:EPPO.
13. Gottwald, T.R., Avinent, L., Llacer, G., Mendoza, A.H.de, and M. Cambra. (1995). Analysis of the spacial spread of sharka (plum pox virus) in apricot and peach orchards in eastern Spain. Plant Disease, 79:266-277.
14. Hammond, J., Puhringer, H., da Camara Machado, A., and M. Laimer da Camara Machado. (1998). A broad-spectrum PCR assay combined with RFLP analysis for detection and differentiation of plum pox isolates. Acta Hort. 472: 483-490.
15. Hartmann, W. (1998). Hypersensitivity – a possibility for breeding sharka resistant plum hybrids. Acta Hort. 472, 429-432.
16. Kalashyan, Y.A., Bilkey, N.D., Verderevskaya, T.D., and E.V. Rubina. (1994). Plum pox potyvirus on sour cherry in Moldova. Bulletin OEPP/EPPO 24:645-649.
17. Kegler, H., and W. Hartman. (1998). Present status of controlling conventional strains of plum pox virus. In: Hadidi, A., Khetarpal, R.K., and H. Koganezawa, eds. Plant Virus Disease Control. Pp. 616-628. APS Press, St. Paul, MN.
18. Kegler, H., Fuchs, E., Grüntzig, M., Schwarz, S. (1998). Some results of 50 years of research on the resistance to plum pox virus. Acta Virol. 42, 200-215.
19. Kerlan, C., and J. Dunez. (1979). Biological and serological differentiation of strains of sharka virus. Annales de Phytopathologie, 11:241-250.
20. Levy, L., and A Hadidi, (1994). A simple and rapid method for processing tissue infected with plum pox potyvirus for use with a specific 3’ non-coding region RT-PCR assay. Bulletin OEEP/EPPO 24:595-604.
21. Llácer, G. and Cambra, M. (1998). Thirteen years of sharka disease in Valencia, Spain. Acta Hort. 472, 379-384.
22. Lopez-Moya, J.J., Fernandez-Fernandez, M.R., Cambra, M., and J.A. Garcia. (1999). Biotechnological aspects of plum pox virus. J. of Biotechnology 1999.
23. Malinowski, T., Zawadzka, B., Ravelonandro, M., Scorza, R. (1998). Preliminary report on the apparent breaking of resistance of a transgenic plum by chip bud inoculation of plum pox virus PPV-S. Acta Virol. 42, 241-243.
24. Myrta, A., Potere, O., Boscia, D., Candresse, T., Cambra, M., & Savino, V. (1998). Production of a Monoclonal specific to the El Amar Strain of Plum Pox Virus. Acta Virologica 42: 248-250.
25. Nemchinov, L., Crescenzi, A., Hadidi, A., Piazzolla, P., and T. Verderevskaya. (1998). Present status of the new cherry subgroup of plum pox virus (PPV-C). In: Hadidi, A., Khetarpal, R.K., and H. Koganezawa, eds. Plant Virus Disease Control. Pp. 629-638. APS Press, St. Paul, MN.
26. Nemeth, M. (1986). Virus, mycoplasma, and rickettsia diseases of fruit trees. Martinus Nijhoff Pub., Dordrecht.
27. Nemeth, M., and M. Kolber. (1983). Additional evidence on seed transmission of plum pox virus in apricot, peach, and plum, proved by ELISA. Acta Hort., 130:293-300.
28. Olmos, A., Dasi, M.A., Candresse, T., and M. Cambra. (1996). Print-capture PCR: A simple and highly sensitive method for the detection of plum pox virus (PPV) in plant tissues. Nucleic Acids Research, 24:2192-2193.
29. Ravelonandro, M., Dunez, J., Scorza, R., Labonne, G. (1998). Challenging transgenic plums expressing potyvirus coat protein genes with viruliferous aphids. Acta Hort. 472, 413-420.
30. Ravelonandro, M., Scorza, R., Bachelier, J.C., Labonne, G., Levy, L., Damsteegt, V., Callahan, A.M., and Dunez, J. (1997). Resistance of transgenic Prunus domestica to plum pox virus infection. Plant Dis. 81, 1231-1235.
31. Scorza, R., Ravelonandro, M., Callahan, A.M., Cordts, J.M., Fuchs, M., Dunez, J., and Gonsalves, D. (1994). Transgenic plums (Prunus domestica L.) express the plum pox virus coat protein gene. Plant Cell Repts. 14, 18-22.
32. Scorza, R., Callahan, A.M., Levy, L., Damsteegt, V., Ravelonandro, M. (1998). Transferring potyvirus coat protein genes through hybridization of transgenic plants to produce plum pox virus resistant plums (Prunus domestica L.). Acta Hort. 472, 421-427.
33. Sherman, W.B., Lyrene, P.M. (1983). Handling seedling populations. p 66-73. IN: Moore, J.N. and Janick, J. (eds) Methods in fruit breeding. Purdue Univ. Press, West Lafayette, IN.
34. Varveri, C., Ravelonandro, M., and J. Dunez. (1987). Construction and use of cloned cDNA probe for the detction of plum pox virus in plants. Phytopathology, 77:1221-1224.
35. Varveri, C., Candresse, T., Cugusi, M., Ravelonandro, M., and J. Dunez. (1988). Use of (32)P-labeled transcribed RNA probe for dot hybridization detection of plum pox virus. Phytopathology, 78:1280-1283.
36. Verhoeven, J.Th.J., de Haas, A.M., Roenhorst, J.W. (1998). Outbreak and eradication of plum pox potyvirus in the Netherlands. Acta Hort. 472, 407-409.
37. Wetzel, T. Candresse, T., Ravelonandro, M., Delbos, R.P., Mazyad, H., Aboul-Ata, A.E., and J. Dunez. (1991a). Nucleotide sequence of the 3’-terminal region of the RNA of the El Amar strain of the plum pox potyvirus. J. of Gen. Virology, 72:1741-1746.
38. Wetzel, T. Candresse, T., Ravelonandro, M., and J. Dunez. (1991b). A polymerase chain reaction assay adapted to plum pox potyvirus detection. J. Virological Methods, 33:355-365.
39. Wetzel, T. Candresse, T., Macquaire, G., Ravelonandro, M., and J. Dunez. (1992). A highly sensitive immunocapture polymerase chain reaction method for plum pox potyvirus detection. J. Virological Methods, 39:27-37
General References
Acta Horticulturae 309 (1992); 386 (1995); 472 (1998).
OEPP/EPPO Bulletin 24 (1994).
Viruses, Mycoplasma, and Rickettsia Diseases of Fruit Trees. (1986). By Maria Nemeth, Martinus Nijhoff Publishers, Dordrecht.
