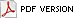
Introduction
Linnaeus named palms “Principes,” the princes of the plant world (15). This noble stature is reflected in the size of some species, with the largest leaves and seeds known in the plant kingdom found in the palm family. The leaves of Raphia regalis can reach 82 feet long (18), and the famous double coconut seed of Lodoicea maldivica can weigh 50 pounds (13). Besides their stature, few plant families provide both food and shelter to people, while at the same time are admired and collected for aesthetic reasons.
Palms belong to a natural but distinctly separate family of plants called the Palmae (synonym = Arecaceae) (18). There are approximately 2700 species of palms in 200 genera that are currently placed in six subfamilies. These plants are highly diverse morphologically and ecologically, and are common in tropical, subtropical and Mediterranean climatic regions of the world. Palms are a source of food and oil; fiber for coir, cord, rope, baskets, hats and mats; rattan used in furniture production; tannin; lumber; thatch for roofing material; wine and other beverages; and for providing a narcotic high (8,13).
Besides this value to humans, palms are also widely employed in interiorscapes, landscapes, and in many national and international tropical gardens, with impressive palm collections housed in greenhouses in temperate climates (e.g., The Palm House at the Royal Botanical Gardens, Kew Gardens in England). In the U.S., primarily in California, Florida and Hawaii, palms are produced for the ornamental industry as potted, greenhouse-grown specimen plants for interior use, or container- and field-grown plants for landscape use. This is in contrast to the plantation fields of palms grown for food, oil and other commercial uses. Ornamental palms are also grown in other parts of the U.S., southern Europe, Central and South America, Japan and Australia.
While palms are naturally distributed on both sides of the equator, human activity, especially by palm enthusiasts, has transported palms to new locations atypical of their native habitat. For example, while Phoenix dactylifera probably originated in the Persian Gulf area and is commonly grown in semi-arid regions for its edible dates (19), it is now ubiquitous throughout subtropical Florida, employed as landscape centerpieces. For palm genera used for food, breeding programs have developed cultivars adapted to the regions in which they are grown. For ornamental palms, however, new cultivars are not bred for new environments, and palms are transported to new locations in which they might not thrive or even survive.
This movement of palms to new environments poses great challenges to ornamental palm growers. Since ornamental palms have a high aesthetic value, leaf spots that are of minor consequence in a plantation are a major problem for the ornamental nursery industry and in the landscape. Likewise, loss of a single specimen palm in the landscape can be extremely costly. However, leaf spots and palm death are not always due to diseases. Disorders, especially nutrient deficiencies, are equally important factors in palm health (4). For example, the primary reason for death of large royal palms (Roystonea regia) in southern Florida is potassium deficiency.
Palms belong to the division of flowering plants known as the monocotyledons (15). Their plant anatomy is more similar to a corn plant than to an oak tree. This anatomical structure has important implications for palm health. Each stem of a palm has a single apical meristem (bud or heart). Once that tissue is damaged, whether from a pathogen, herbicide, nutritional deficiency, environmental factors, or mechanical damage, death is likely. This is especially true for single-stem palms. Since palm stems have no vascular cambium, they are essentially devoid of secondary growth. Therefore, palms cannot repair injuries to their stems, and diligent effort must be made to prevent injuries that provide opportunities for insect or pathogen invasion of the trunk.
The remaining portion of this article focuses on two lethal diseases of ornamental palms in the landscape -- Ganoderma butt rot, which is a significant problem in Florida, and Phytophthora bud rot, which is a problem where ever palms are grown.
Ganoderma Butt Rot (Basal Stem Rot)
This disease has many names around the world, but it is always a lethal disease of palms. The disease is referred to as Ganoderma butt rot in the continental U.S., and thus far is limited to the southeastern region of the country (6). In general, the natural geographic range of the disease in the U.S. matches that of the indigenous sabal palm (Sabal palmetto). However, as infected, but non-symptomatic, mature palms are moved out of this region for horticultural landscape purposes, the disease has the potential to spread. In tropical regions that grow oil palm (Elaeis guineensis) as a plantation crop or in wild groves, the disease is referred to as basal stem rot (7). It is considered the most serious disease of oil palm in southeast Asia, especially in Malaysia and Indonesia, but also in Thailand and Papua New Guinea.
Efforts over the past century to identify and classify species of Ganoderma can best be described as “chaotic.” However, recent molecular systematic studies indicate there may be some uniformity concerning the two primary species pathogenic on palms, G. zonatum in the U.S. and G. boninense in southeast Asia (11).
The primary foliar symptoms of Ganoderma butt rot include a mild to severe wilt (Fig. 1), reduced growth, overall off-color foliage (not chlorotic, just paler green than normal), and older fronds that are chlorotic or necrotic (Fig. 2) that eventually droop (Fig. 3). By the time foliar symptoms develop, usually over half of the lower internal stem tissue (trunk) has been killed by the fungus.
| |
 |
|
 |
|
| |
Fig. 1. Sabal palmetto with wilted and necrotic leaves caused by Ganoderma zonatum infection. (Courtesy of M. L. Elliott) |
|
Fig. 2. Comparison of Phoenix canariensis without (right) and with (left) Ganoderma butt rot. (Courtesy of M. L. Elliott) |
|
| |

Fig. 3. Drooping of foliage due to infection of Syagrus romanzoffiana by Ganoderma zonatum. (Courtesy of M. L. Elliott) |
|
Cross-sections of the lower trunk show an infected central area that is usually soft and distinct in color from healthy tissue surrounding it (Fig. 4). Serial cross-sections of infected palm stem tissue show a disease progression pattern that is cone-shaped, with the greatest portion of diseased trunk at the soil line and the disease progressing upwards in the center of the trunk (Figs. 5 and 6). The exact infection point is unknown. The fungus does not normally extend more than 5 feet up into the palm trunk. Once the fungus has moved to the outside edge of the palm stem, basidiocarps (conks) may form external to the trunk, releasing basidiospores (Fig. 7).
 |
|
 |
|
Fig. 4. Cross-section of palm trunk illustrating internal wood rot caused by Ganoderma zonatum. (Courtesy of M. L. Elliott) |
|
Fig. 5. Cross-sections of lower trunk infected with Ganoderma zonatum. Top-left section is bottom section at soil line (section 1) and remaining sections progress up the trunk. (Courtesy of M. L. Elliott) |
 |
|
 |
|
Fig. 6. Growth of mycelium on infected trunk sections after incubation in moist chamber. White growth is mycelia of Ganoderma zonatum. (Courtesy of M. L. Elliott.) |
|
Fig. 7. Three phases of basidiocarp development of Ganoderma zonatum: top is beginning stage; lower right is maturing stage but no spore release; lower left is older, mature conk after spore release. (Courtesy of M. L. Elliott.) |
The basidiocarp initially begins as a white flat mass that is irregular to circular in shape. As it develops, it begins to expand outward from the trunk growing into a ball shape, but it remains white and is relatively soft when touched (Fig. 7). As the basidiocarp matures, it protrudes from the trunk, eventually forming a distinct, shelf-like structure that is quite hard with a glazed reddish-brown top surface and a white undersurface that often swells at the edge (Fig. 7). The top surface of G. zonatum has distinct zones of growth, hence the species name (Fig. 8). The basidiocarp of G. zonatum is directly attached to the trunk (sessile). The underside of the basidiocarp is composed of microscopic pores or tubes where the basidiospores are formed and released. The white undersurface of the basidiocarp becomes a brownish-red color once the basidiospores are released from a mature basidiocarp, due to the color of the spores en masse.
| |

Fig. 8. Mature basidiocarp of Ganoderma zonatum. (Courtesy of M. L. Elliott.) |
|
The death of 58 palm species due to G. zonatum has been documented in Florida (6). While the majority of these palms were planted as ornamentals in the landscape, even naturalized sabal palms have been killed by the fungus. All palm species having woody trunks are considered potential hosts of this fungus.
Although research on Ganoderma butt rot is still quite limited, the diversity and isolation of disease occurrence locations in the southeastern U.S. supports the theory that basidiospores act as the primary means of spread. Recent research in southeast Asia on G. boninense also implicates the role of spores in the spread of the pathogen (10). Confirmation of Ganoderma butt rot cannot be made until the basidiocarp forms on a standing palm, or when an unhealthy tree is cut down and the symptomatic internal trunk rotting is observed. There are currently no reliable diagnostic methods for isolating and identifying G. zonatum from trunk tissue or soil.
In the U.S., no common environmental conditions, soil types, or landscape management practices have been observed that favor the development of Ganoderma butt rot (6). The disease has been observed in natural settings (palms never transplanted) and in highly-maintained, transplanted landscapes. No effective controls are known for Ganoderma butt rot.
Phytophthora Diseases
Several species of Phytophthora cause major diseases of palms throughout the world, with bud (heart) rot the most common and devastating disease. A lethal bud rot of several species of palms is caused by Phytophthora palmivora (Figs. 9 and 10). On coconut (Cocos nucifera) and other palms, an early symptom is the discoloration of the spear leaf (youngest unexpanded leaf). As the infected spear leaf unfolds, large, dark brown frond rots are produced that progress into the leaf base killing the bud. Older leaves are supported by the vascular system in the older part of the trunk and remain green for months. Eventually all leaves are lost, leaving only bare trunks (5). Similarly, heart or bud rot of coconut caused by P. katsurae is signaled by the death of the youngest leaf (Fig. 11). When the young leaf dies, bud rot is already advanced (Figs. 12 and 13). There is a progressive loss of young leaves, producing trees with only a skirt of old leaves and no young leaves (Fig. 14), followed by death of the palm.
| |
 |
|
 |
|
| |
Fig. 9. Phytophthora bud rot on Chamaedorea seifrizii. (Courtesy of T. K. Broschat.) |
|
Fig. 10. Phytophthora bud rot on Trithrinax acanthocoma. (Courtesy of N. A. Harrison.) |
|
|
 |
|
 |
|
|
Fig. 11. Death of spear leaf of young Cocos nucifera infected with Phytophthora katsurae. (Courtesy of J. Ooka.) |
|
Fig. 12. Internal rot of leaf base of Cocos nucifera caused by Phytophthora katsurae. (Courtesy of J. Ooka.) |
|
| |

|
|
 |
|
| |
Fig. 13. Close-up of bud (heart) rot of Cocos nucifera caused by Phytophthora katsurae. (Courtesy of J. Ooka.) |
|
Fig. 14. Advanced bud (heart) rot of Cocos nucifera, with only older fronds remaining, caused by Phytophthora katsurae. (Courtesy of J. Ooka.) |
|
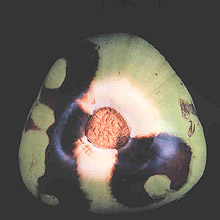
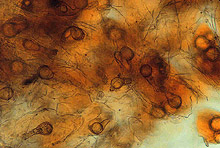
Large numbers of coconut palms used in the landscape in Hawaii have been killed by P. katsurae (16,17). Premature nut drop is the earliest symptom of this devastating disease. Dropped nuts are of various sizes, but more often are large, nearly mature nuts with irregular brown to black areas beginning at the stem end of the nut. These darkened areas of infected husk form around circular, green, uninfected islands (Fig. 15). Infections of young nuts and early infections of mature nuts result in the formation of darker green, water-soaked areas that may appear oily (Fig. 16). Internally, the husk is dark brown in young diseased fruits, while mature diseased fruits have reddish brown husks (Fig. 17). Secondary invasion by Thielaviopsis paradoxa blackens infected husks. Phytophthora katsurae forms numerous oospores in host tissue (Fig. 18) and in culture. Oogonia produced in culture are distinctive with several protuberances on each oogonium and a long funnel shaped base (Fig. 19). In host tissue, most oogonia do not produce protuberances (Fig. 20), and this has possibly led to identity confusion with Phytophthora heveae.
 |
|
 |
|
Fig. 15. Green uninfected Cocos nucifera fruit tissue surrounded by necrotic rot caused by Phytophthora katsurae. (Courtesy of J. Y. Uchida.) |
|
Fig. 16. Developing Cocos nucifera fruit lesion caused by Phytophthora katsurae. (Courtesy of J. Y. Uchida.) |
|
 |
|
 |
|
Fig. 17. Internal rot of Cocos nucifera fruit caused by Phytophthora katsurae. (Courtesy of J. Y. Uchida.) |
|
Fig. 18. Oospores of Phytophthora katsurae from infected Cocos nucifera fruit. (Courtesy of J. Y. Uchida.) |
| |
 |
|
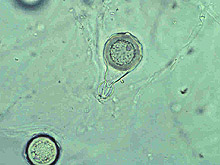 |
|
| |
Fig. 19. Oospore of Phytophthora katsurae formed in culture. Note long oogonial base and oogonial protuberances. (Courtesy of J. Y. Uchida.) |
|
Fig. 20. Oospore of Phytophthora katsurae formed in host tissue and lacking protuberances. (Courtesy of J. Y. Uchida.) |
|
Phytophthora palmivora also causes early nut drop of coconut in Southeast Asia and the Caribbean. These nuts are borne on infected rachilla (inflorescence stalks), and stem ends attached to the diseased rachilla are infected. The nuts have brown infected husks internally, while a brown rot spreads from the stem end externally. A few months after nuts are lost, two to three leaves in the middle of the canopy will droop (Fig. 21) as the infection decays the leaf base and the weight of the leaf causes it to bend and droop. Trees are killed following bud rots.
| |

Fig. 21. Drooping leaves of mature Cocos nucifera caused by Phytophthora bud rot. (Courtesy of N. A. Harrison.) |
|
Phytophthora palmivora also attacks Mexican and California fan palms, Washingtonia robusta and W. filifera respectively, causing bud rot and destruction of young plants (Fig. 22). Infected plants exhibit a general decline in plant growth, with the spear leaf becoming pale green then greenish-brown followed by light brown, as leaves become desiccated (3). The bases of infected leaf stems develop tan-colored necrotic rots with brown margins, and plants are slowly killed as buds are entirely rotted (Fig. 23).
 |
|
 |
|
Fig. 22. Phytophthora bud rot on Washingtonia sp. in wet area of field. (Courtesy of T. K. Broschat.) |
|
Fig. 23. Cross-section of Washingtonia sp. stem infected with Phytophthora palmivora. (Courtesy of T. K. Broschat.) |
Leaf spots and severe blights of parlor palm (Chamaedorea elegans) and golden palm (Dypsis lutescens) are caused by P. palmivora (Fig. 24). Leaf spots begin as small, gray-green irregular, circular to elongate lesions that expand to large spots and blights with thin lamina, tan to gray centers and black to dark brown edges. Large spots are irregular in shape and somewhat vein-limited. The spear leaf may be infected, and part or all of the leaf and bud are rotted. Young palms develop leaf lesions that advance to the bud and kill the plants.
| |

Fig. 24. Leaf spots of Chamaedorea elegans caused by Phytophthora palmivora. (Courtesy of C. Kadooka.) |
|
Phytophthora nicotianae (synonym = P. parasitica) causes seedling blight of golden palm (12). The disease begins as small brownish flecks that expand to angular, irregularly shaped leaf spots and finally into large blighted areas. Young leaves are very susceptible, and infections of the unfurled leaves that progress to the terminal bud kill the young plant. Phytophthora nicotianae also attacks California fan palm, causing trunk and collar rots. The disease appears to begin from wounds caused by leaf removal at or near the soil line. Although this disease can be reproduced by inoculations of the lower trunk, the bud and entire stem is eventually rotted on some plants. Thus, P. nicotianae should also be considered as a possible cause of bud rots.
In Florida, Phytophthora arecae was confirmed as a root rotting pathogen of Chamaedorea seifrizii x erumpens. Root rots were characterized as blackened, water soaked tissue but were not severe, affecting less than 15% of the root system (14). Two other Phytophthora diseases are a stem and collar rot of Mexican fan palm caused by a Phytophthora species, believed to be P. cryptogea (or P. drechsleri) (9) and a petiole rot of golden palm caused by P. tropicalis in Hawaii.
Phytophthora palmivora is worldwide in distribution in tropical and warm temperate regions. This pathogen has been reported on palms from over 20 countries as well as from California, Florida, Hawaii, and Puerto Rico. The fungus has a broad host range and has been reported to attack over 25 palm species, but it is characterized by host specificity among some isolates. It is the most commonly reported Phytophthora species on palms (1).
Phytophthora nicotianae causes stem, leaf and root rots on parlor palm, bamboo palm (C. erumpens), reed palm (C. seifrizii), red sealing wax palm (Cyrtostachys renda), golden palm, Macarthur palm (Ptychosperma macarthurii), thatch palm (Thrinax sp.), and Mexican fan palm (1). Phytophthora katsurae has been isolated from coconut palm in Hawaii, and, although devastating on this palm, it has not been isolated from other palms or other hosts in Hawaii. Phytophthora tropicalis has been isolated from petiole rots of golden palm, is widely distributed in the tropics, and has been isolated from numerous plants in Hawaii and in the tropics (2). Unidentified Phytophthora species are reported to be associated with wilts, and stem, bud, crown, and root rots of over 25 other palm species (1).
Disease cycles depend on the host and Phytophthora species involved. Phytophthora palmivora produces sporangia that are splashed to new hosts or healthy tissue, resulting in bud rots that begin at the top of the canopy. In India, the incidence of bud rot of coconut is correlated with a 2- to 6-month hurricane/severe storm period. Similar observations have been made in Hawaii, where frequency of coconuts infected with P. katsurae increased after major storms or hurricanes. These storms also damage coconut crowns, exposing the palm to pathogen invasion.
Phytophthora drechsleri and P. nicotianae produce non-deciduous sporangia and infect the lower sections of stems (trunks). As infections form in the upper plant canopy, greater movement of these pathogens can occur. Phytophthora nicotianae often produces sporangia on host tissue, which are released and become airborne (splashed). Species that form chlamydospores (e.g., P. palmivora, P. nicotianae, and P. tropicalis) or oospores (P. katsurae) will survive in the soil or potting mix for many months. Chlamydospores of P. palmivora have been found in decomposing coconut tissue months after plant kill. Insects, birds, slugs, snails, and nursery employees can carry spores from diseased to healthy plants. Previously used media, pots, trays, and tags can all harbor Phytophthora spores. Good drainage is also important as zoospores, the swimming spores formed by sporangia, will move from plant to plant as long as water is present. Wet foliage will encourage zoospore formation, and these zoospores are believed to swim to stomatal openings (tiny pores on the surface of leaves and stems for gas exchange) and penetrate healthy leaves. Zoospores have been found in water on benches, ditches, puddles, and streams.
In the United States some evidence exists that commercial tree trimming operations spread diseases. The common practice of severely pruning palm trees to lengthen the time between trimmings exposes the plant to pathogens. The boot spikes, saw blades, and other pruning tools can become contaminated with diseased tissue or spores, which are transferred directly to the cut surfaces of healthy palms.
In the ornamental industry, most palm species are propagated by seed, thus procedures for clean seed culture must be followed. Seedling diseases caused by Phytophthora spp. can be prevented by use of clean seeds, new potting media, new pots, and new trays.
Moisture control is extremely important for diseases caused by Phytophthora spp. Employ cultural measures to reduce excess moisture, such as increasing the distance between plants, pruning old growth, trimming surrounding vegetation, increasing air movement, irrigating by drip as opposed to overhead watering, and irrigating in the morning to reduce wet foliage at night. Practice sanitation by trimming severely diseased leaves and removing diseased petioles and sheaths. Rogue and discard all severely diseased plants, especially plants with bud rots. If only a few potted plants are mildly infected, isolate them from the rest of the crop and make efforts to eliminate the disease by moisture reduction and fungicide applications. Phytophthora nicotianae and P. palmivora are pathogens of many ornamental hosts, and both may persist in the greenhouse environment for years. Apply chemical fungicides, as necessary, to prevent disease. Products specific for Phytophthora, such as fosetyl-aluminum, metalaxyl, propamocarb or mefenoxam, will prevent disease or may eliminate early infections. Follow label directions when using any pesticide.
Attempts to save coconut trees with heart (bud) rot caused by P. katsurae have been unsuccessful when palms were treated with metalaxyl drenches, but injections of metalaxyl and fosetyl-aluminum have been somewhat effective. Disease prevention is crucial, as infected trees are virtually impossible to save except for those with minor trunk lesions. In Hawaii, all trees infected with P. katsurae have died. The injection of phosphorous acid formulations in commercial operations in Hawaii, may have prevented infections by Phytophthora, but the drilled hole made at the injection site allows Thielaviopsis and other wound pathogens entry deep into the trunk, with trunk diseases resulting in some locations.
Additional Resources
Palm and Cycad Societies of Florida
Palms of the World
International Palm Society;
publishes the journal Palms, formerly Principes
Palm and Cycad Society of Australia
Fairchild Tropical Garden
National Tropical Botanical Garden
Montgomery Botanical Center
Literature Cited
1. Alfieri, S. A., Langdon, K. R, Kimbrough, J. W, El-Gholl, N. E., and Wehlburg, C. 1994. Diseases and Disorders of Plants in Florida. Florida Dept. Agric. & Consumer Serv., Div Plant Industry. Bull. 14.
2. Aragaki, M., and Uchida, J. Y. 2000. Morphological distinctions between Phytophthora capsici and P. tropicalis sp. nov. Mycologia 93:137-145.
3. Atilano, R. A. 1982. Phytophthora bud rot of Washingtonia palm. Plant Dis. 66:517-519.
4. Broschat, T. K., and Meerow, A. W. 2000. Ornamental Palm Horticulture. University Press of Florida, Gainesville, FL.
5. Corbett, M. K. 1959. Diseases of the coconut palm. Principes 3:117-120.
6. Elliott, M. L., and Broschat, T. K. 2001. Observations and pathogenicity experiments on Ganoderma zonatum in Florida. Palms 45:62-72.
7. Flood, J, Bridge, P. D., and Holderness, M., eds. 2000. Ganoderma Diseases of Perennial Crops. CABI Publishing, Wallingford, U.K.
8. Howard, F. W., Moore, D., Giblin-Davis, R. M., and Abad, R. G. 2001. Insects on Palms. CABI Publishing, Wallingford, U.K.
9. Keim, R., Klure, L. J. and Zentmyer, G. A. 1979. Collar rot of Washington palms in containers. Plant Dis. Reptr. 63:718-720.
10. Miller, R. N. G., Holderness, M., Bridge, P. D., Chung, G. F., and Zakaria, M. H. 1999. Genetic diversity of Ganoderma in oil palm plantings. Plant Path. 48:595-603.
11. Moncalvo, J.-M. 2000. Systematics of Ganoderma. Pages 23-45 in: Flood, J, Bridge, P. D., and Holderness, M., eds. Ganoderma Diseases of Perennial Crops. CABI Publishing, Wallingford, U.K.
12. Nagata, N. M. and Aragaki, M. 1989. Etiology and control of Phytophthora leaf blight of golden-fruited palm. Plant Dis. 73:661-663.
13. Neal, M. C. 1965. In Gardens of Hawaii. Bishop Museum Press, Honolulu, HI.
14. Ploetz, R. C. and Mitchell, D. J. 1989. Root rot of bamboo palm caused by Phytophthora arecae. Plant Dis. 73:266-269.
15. Tomlinson, P. B. 1990. The Structural Biology of Palms. Clarendon Press, Oxford, U.K.
16. Uchida, J. Y., Aragaki, M., Ooka, J. J., and Nagata, N. M. 1992. Phytophthora fruit and heart rot of coconut. Plant Dis. 76:925-927
17. Uchida, J. Y., Ooka, J. J., Nagata, N. M., and Kadooka, C. Y. 1992. A new Phytophthora fruit and heart rot of coconut. Haw. Inst. Trop. Agric. Hum. Resour, Res. Ser. No. 138. Coll. Trop. Agric. Hum. Res. Pub., Univ. of Hawaii.
18. Uhl, N. A., and J. Dransfield. 1987. Genera Palmarum. Allen Press, Lawrence, KS.
19. Zaid, A., ed. 1999. Date Palm Cultivation. FAO Plant Prod. Prot. Pap. No. 156.
