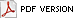
The first use of methyl bromide as a soil fumigant occurred in France in the 1930s (7). Since its discovery and implementation, methyl bromide has been consistently effective for control of nematodes, fungi, insects and weeds and has been used on more than 100 crops worldwide. Methyl bromide’s high vapor pressure allows for rapid and thorough distribution through the soil, enhancing its effectiveness as a fumigant. The high vapor pressure also facilitates a relatively short plant-back interval and gives growers a great degree of flexibility. For nearly four decades, methyl bromide has been the fumigant most heavily relied upon for pre-plant soil treatment for the production of vegetables and ornamentals. The world’s largest consumer of methyl bromide is the United States, where the majority of use (83%) is for pre-plant soil fumigation (119). Other uses include post-harvest treatment of stored commodities (11%) and structural fumigation (6%). Based on 1997 U.S. consumption records, 36% of pre-plant methyl bromide use took place in Florida crop production systems, with strawberry, pepper and tomato accounting for 9, 23, and 62% of the soil fumigation uses in the state (84). Methyl bromide is considered essential for the production of eggplant, pepper, strawberry, watermelon and tomato in many locations (41,42,124). The nursery industry accounts for nine percent of the U.S. pre-plant consumption of methyl bromide for the production of potted plants, cut flowers, ornamental nursery plants, fruit and tree nursery plants, sod, bulbs, strawberry and vegetable transplants (118). Within the floriculture industry, there are few statistics on methyl bromide use by individual crops, although certain segments such as the field production of chrysanthemum, caladium, and gladiola rely heavily on its use as a soil fumigant (57).
Methyl Bromide and the Atmosphere
The area of the atmosphere from the Earth’s surface to 10 km in altitude is referred to as the troposphere. The next layer up is referred to as the stratosphere and lies between 10 and 50 km. The decrease of Antarctic stratospheric ozone began in the early 1970s, and by the mid-1980s scientists confirmed that ozone levels measured in October were as much as 35% lower than levels recorded during the 1960s (33). By 1994, Antarctic ozone levels had decreased by as much as 50% (123). The amount of overhead ozone (total ozone) is measured in Dobson Units (DU). In the 1950s-1960s, average Antarctic total ozone was approximately 300 DU, but had decreased to 100 DU by the early 1990s. Levels of 220 DU or less define the area referred to as the ozone hole. This value is used because no values this low were ever recorded prior to the 1980s (86). The hole over Antarctica begins to form in early August, reaching maximum size in late-September. Ozone levels during this time can drop by more than 50% (33). The hole begins to disappear by December. The seasonal average size of the hole has reached as much as 28 million km2, with a typical average of 25 million km2 (86).
Ozone depletion since the 1970s has been attributed to an increase in chlorine and bromine in the stratosphere (125). Polar ozone loss is caused by heterogenous chemical processes in which chlorine and bromide molecules are converted to reactive forms by chemical reactions that take place on particles found in polar stratospheric clouds. This occurs over Antarctica due to some unique conditions. Temperatures over the polar vortex region reach -90˚C in early August. The presence of the polar night jet prevents mixing of warmer, ozone-rich air from the mid-latitudes. These cold temperatures allow for the formation of the polar stratospheric clouds, upon which these reactions are taking place. Reactions involving bromide are considered to contribute 50% of the loss of ozone over Antarctica each year (18). Polar stratosphere clouds also form in the Arctic stratosphere, resulting in Arctic ozone losses. However, Arctic temperatures are warmer than Antarctic temperatures, resulting in smaller losses. Although the losses over the South Pole have been most pronounced, losses between 5-10% have also been detected in the mid-latitudes (123).
While chlorine exists in the stratosphere at approximately 3.5 ppb, bromine is present at 15-20 ppt (125). Reactions involving BrO are approximately 50 times faster than reactions involving ClO, therefore the smaller amount of bromine in the stratosphere still represents a significant danger to the ozone layer (22). While methyl bromide is abundant in the atmosphere, soil fumigation is just one source of the material. Other sources include, emissions from leaded gasoline (113) and biomass burning (6), as well as natural sources such as oceans (80), salt marshes (102), rice paddies (101) and litter decomposition. It is estimated that approximately 30% of methyl bromide emissions result from soil fumigation (126).
Based on its ability to deplete ozone (referred to as the ozone depletion potential (ODP)) of 0.38 and lifetime of 0.7 years, methyl bromide has been classified as a Class 1 stratospheric ozone depletor (125). Based on the Vienna Convention on the Protection of the Ozone Layer, signed in 1985, which established the legal framework for addressing anthropogenic sources of ozone depleting substances, the Montreal Protocol was established in 1987, in which the parties established guidelines for the reduction of ozone depleting substances. An amendment to the protocol in 1992 identified methyl bromide as a substance of concern and established a phase-out program in developed countries of 25, 50, 75, and 100% reductions from the 1991 domestic production level in 1999, 2001, 2003 and 2005 respectively. Developing countries were given a modified schedule in which a 20% reduction from 1995-1998 would be followed by a 100% reduction by 2015 (3). Initially, in 1994, the US EPA determined that under the Clean Air Act, this fumigant must be phased out by 2001. Provisions were made that allowed the U.S. to conform to the conditions set forth in the Protocol.
It does appear that the Montreal Protocol is having a positive impact on the measurable amounts of chlorine and bromine in the atmosphere. Although a reduction in bromine (79) and methyl bromide (127) in the troposphere and a reduction in chlorine in the stratosphere has been documented since the adoption of the Montreal Protocol (125), there is not enough data to conclude that there has been a decrease in stratospheric bromine during this time period (P.A. Newman, NASA, personal communication). The impact on the ozone hole itself is even more difficult to document. Due to annual variations in Antarctic stratospheric temperatures, the ozone hole changes, sometimes dramatically, from year to year, occasionally leading to the conclusion that hole in the ozone layer is reducing (Fig. 1). Colder temperatures lead to more chlorine activation, which results in greater chlorine- and bromine-induced ozone loss. Minor reductions in the ozone hole that could be attributed to reductions of chlorine and bromine in the stratosphere are likely to be masked by the variation resulting from these temperature changes. According to Newman et al., (86) "Only a few percent decrease from maximum size will have occurred by 2015 solely as a result of decreasing halogens…" New coupled chemistry climate models predict recovery of ozone by about 2050 (86).
| |
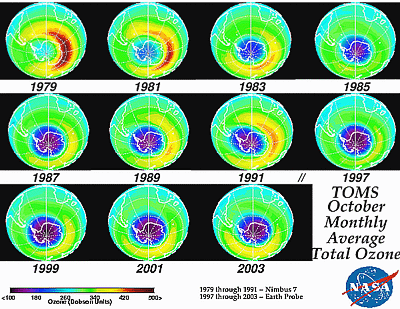
Fig. 1. Total Ozone Mapping Spectrometer (TOMS) October monthly average ozone measurements over Antarctica from 1979 to 2003. Data from 1992-1996 were not collected as the transition from Nimbus 7 to Earth Probe took place. Graphic courtesy of David Larko, NASA. |
|
Status of Methyl Bromide Critical Use Exemptions (CUE)
The CUE process. In 1997, the Parties to the Montreal Protocol recognized a justifiable need for transitional access to methyl bromide, and adopted a formal decision to allow limited critical use exemptions (CUE). Exemptions were to be granted only when the following criteria were met:
1. Failure to provide access to methyl bromide would result in a significant market disruption;
2. There were no technically and economically feasible alternatives available to an applicant that were acceptable from environmental and human health standpoints;
3. The applicant had taken all feasible steps to minimize their use of methyl bromide and the associated emissions; and
4. Appropriate efforts were being made to evaluate, commercialize and register alternatives to methyl bromide for use by the applicant.
The exemption application process is extremely rigorous. Detailed information is required from each applicant, including comprehensive information on the impact of alternatives on crop yields and profit margins, and a description of efforts undertaken to develop, register, and apply new alternatives. In order to assist growers in the application process, in March 2001, the U.S. Environmental Protection Agency (EPA) began meeting with stakeholders to inform them of their understanding of requirements under the Montreal Protocol for attaining a CUE.
The U.S. State Department then submitted one critical use nomination (CUN) on behalf of all U.S. methyl bromide users to the Ozone Secretariat of the United Nations Environmental Programme (UNEP), which is the administrative oversight body for the Montreal Protocol. The U.S. nomination consisted of sectors representing the industries that submitted CUE applications. The Ozone Secretariat then forwarded all CUN’s to the Montreal Protocol's technical assessment bodies, on behalf of applicants. The technical assessment bodies are the Technology and Economic Assessment Panel (TEAP) and the Methyl Bromide Technical Options Committee (MBTOC). These bodies then made recommendations on the applications to the Parties to the Protocol. The Parties then determined whether or not to approve each application.
Critical use nominations (CUN) currently in effect or under consideration. In May 2002, the initial Federal Register Notice was published announcing the 120-day data collection and petition preparation period. The list of alternatives required to be addressed by applicants came from an official list established at the international level, which was revised to reflect products registered in the U.S.
In 2002 and 2003, the U.S. nominated 16 crops/uses for a CUE for methyl bromide use in 2005 and 2006. These were commodity storage, cucurbits, eggplant, food processing, forest tree seedling nursery, ginger, nursery seed bed trays, orchard nursery, orchard replant, ornamental nursery, pepper, strawberry, strawberry nursery, sweet potato, tomato, and turf grass. For 2005, the Parties authorized 35% of the U.S. 1991 baseline for a critical use exemption. These amounts were circulated in a final rulemaking published in the Federal Register in December 2004. See the Authors' List of Related Links for more information on the final ruling.
In 2004, nominations were made for methyl bromide use in 2006. The U.S. nominated a total of 37% of the 1991 baseline. The Parties authorized 29% with 10% to be assessed at a one-day extraordinary meeting of the Parties in July 2005. This nomination covers exemptions for 17 crops or uses, including tomatoes, strawberries, peppers, cucurbits, orchard replant, forest nurseries, turf, and post-harvest uses.
The U.S. submitted its third CUN for review and authorization on January 31, 2005. The current request is for calendar year 2007 and covers 15 agricultural sectors including tomatoes, strawberries, peppers, cucurbits, orchard replants, turf, forest nurseries, and post-harvest uses. This request will be considered by the Parties at their 17th Meeting at the end of 2005. In the nomination for 2007, the U.S. is requesting 29 percent of 1991 baseline amounts. See the Authors' List of Related Links for additional information on specific commodity sectors and copies of all U.S. Nominations.
Quarantine and preshipment use (QPS). QPS uses of methyl bromide are not subject to the Montreal Protocol's 2005 phase-out, and are defined by the Protocol. Quarantine applications are "those used to prevent the introduction, establishment and/or spread of quarantine pests or to ensure their official control where; (i) official control is that performed by, or authorised by, a national plant, animal or environmental protection or health authority, and (ii) quarantine pests are pests of potential importance to the areas endangered thereby and not yet present there, or present but not widely distributed and being officially controlled." Pre-shipment applications are "those non-quarantine applications applied within 21 days prior to export to meet the official requirements of the importing country or existing official requirements of the exporting country." Official requirements are those which are performed by, or authorized by, a national plant, animal, environmental, health or stored product authority. See the Authors' List of Related Links for additional information on the process for exempting quarantine and preshipment applications.
There is also a contingency for emergency use of methyl bromide that is distinct from QPS uses. After the phase-out of methyl bromide, a country will be allowed to use up to 20 metric tons per year for emergency use, and apply for approval after the use.
National management strategy (NMS). In March 2004, at the Extraordinary Meeting of the Parties in Montreal, conditions for granting and reporting CUE were determined. In addition, they also requested that each Party making a nomination after 2005 submit a National Management Strategy (NMS) for phase-out of critical uses to the Ozone Secretariat before February 1, 2006. The NMS is to address:
1. the avoidance of any increase in methyl bromide consumption except for unforeseen circumstances
2. the encouragement of the use of alternatives through expedited procedures, to develop, register and deploy technically and economically feasible alternatives
3. provision of information for each use for which a nomination is planned on the potential market penetration of newly deployed alternatives, to bring forward the time when methyl bromide consumption for such uses can be reduced or eliminated
4. promotion of the implementation of measures which ensure that any emissions of methyl bromide are minimized
5. demonstration of how the NMS will be implemented to promote the phase-out of methyl bromide as technically and economically feasible alternatives are available, describing the progress of each Party with respect to research programs and adoption of alternatives.
Allocation rule. In late December 2004, the EPA finalized a rule to create the CUE. A framework for granting "critical use allowances" to producers and importers of methyl bromide has been designed to allow production and importation of up to 30% of 1991 methyl bromide baseline for use in 2005. An additional, 5% of the 1991 baseline can be used from "critical stock allowances" which allow holders to sell methyl bromide to critical users from stocks that were manufactured or imported before January 1, 2005. Users of methyl bromide (excluding those for QPS applications) must meet specified criteria and their application must be designated as a critical use in order to be able to purchase methyl bromide from their supplier. Users must certify, under penalty of law, that they are approved critical users. For instance, in Florida, limiting conditions which qualify a use as critical include the presence of karst topography (land above caves or underground channels) or a reasonable expectation of moderate to high infestation of nutsedge (Cyperus rotundus or C. esculentus). See the Authors' List of Related Links for additional information.
Methyl Bromide-Dependent Crops: Florida Tomato and Pepper
Production. Florida’s cash receipts for bell peppers(Capsicum annuum) ranked first and tomato (Lycopersicon esculentum) receipts second in the country based on 2002 data. These crops account for 3.2 and 7.4 % of Florida’s total agricultural receipts respectively. In the 2002-2003 growing season, bell peppers were produced on 7,203 ha with a total value of approximately $178 million. In 2003-2004, planted area increased slightly to 7,487 ha with cash receipts totaling $218.4 million. Tomato acreage dropped slightly from a 2002-2003 total of 17,523 ha to 17,159 ha in 2003-2004, with a subsequent drop in cash receipts from $550.6 million to $500.5 million. Based on 2004 receipts, Florida tomato and pepper crops have an annual value exceeding $725 million (35).
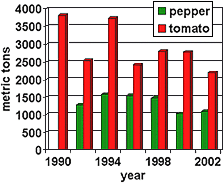
Commercial vegetable production takes place throughout Florida in climates ranging from humid sub-tropical to tropical (116) (Fig. 2). Average annual rainfall ranges from approximately 40-65 inches per year. Soils are sandy, have minimal organic matter, and are relatively low in fertility. Conventional growers use a "raised bed-plastic mulch" production system that was developed in the 1960s (42,59,98) and has served a pivotal role in the economic success of southeastern U.S. tomato and pepper production (8,41). This system is dependent upon the use of a polyethylene-mulch covered raised bed, on which plants are staked and tied as they grow (95). Growers use either drip or seepage irrigation, which is composed of a system of irrigation ditches in which water levels are raised and lowered to control soil moisture levels in the field. Beds are fumigated with a mixture of methyl bromide and chloropicrin. Fumigants are shank injected using chisels spaced 30 cm apart as the soil is being pulled up to form the beds (Fig. 3). The beds are then covered immediately with polyethylene plastic, which functions as mulch. 2002 estimates of methyl bromide use indicate use in bell pepper at 1101 metric tons and 2207 metric tons in tomato (Fig. 4).
| |
Fig. 2. A. Pepper commercial production areas. B. Tomato commercial production areas and C. Commercial strawberry production areas. Figure modified from USDA, Crop Profiles (24). |
|
 |
|
 |
|
Fig. 3. In-bed applications of methyl bromide with application of plastic mulch in the "raised-bed, plastic mulch culture system." This picture also illustrates the irrigation ditches described above. |
|
Fig. 4. Historical methyl bromide use for the production of peppers and tomatoes in Florida derived from NASS (84). |
Important pests in Florida tomato and pepper production. There is a plethora of pests that impact the production of Florida tomatoes and peppers. Major soilborne pests of tomato and pepper include root-knot nematode, a number of fungal plant pathogens which vary according to the production area, and multiple weeds. On tomato, Fusarium wilt, caused by Fusarium oxysporum f. sp. lycopersici and Fusarium crown rot (Fusarium lycopersici f. sp. radiscis-lycopersici) are found throughout the state (16,111). Fusarium crown rot is more of a problem when the soil temperatures are cool (100), while Fusarium wilt is favored by higher temperatures and is very common in sandy soils. Races 1, 2 and 3 of this pathogen are present throughout the state of Florida (16). Symptoms of Fusarium wilt on mature plants often begin as a leaf-yellowing that occurs on one side of the plant and also on only one side of a leaflet. The plant will then begin to wilt until the vascular system is completely compromised and the plant dies (58) (Fig. 5). There are some commercially available tomato hybrids that have been bred for resistance to race 3 and are suitable for the Florida production region.
| |

Fig. 5. Epidemic of Fusarium wilt caused by Fusarium oxysporum f. sp. lycopersici race 3. |
|
Verticillium wilt and Corky brown root rot (Pyrenochaeta lycopersici) are pathogen problems that are unique to the Dade county tomato production area, but are of considerable concern (73).

Fig. 6. The small pepper plant in the center displays symptoms typical of infection with Pythium aphanidermatum and P. myriotylum. The importance of these pathogens in a post-methyl bromide production system is just emerging. |
|
Some pathogen problems are common to both tomatoes and peppers. Damping-off, caused by Pythium spp. and Rhizoctonia spp., during the early stages of growth is a significant problem in both crops. Recently, Pythium spp. have been associated with disease symptoms in mature plants and identified as a production limitation. Symptoms of this problem are similar to those associated with low fertility, including stunting, chlorosis, and reduced vigor (Fig. 6). The greatest amount of root damage in tomato is caused by P. aphanidermatum and P. myriotylum, although they do not cause plant mortality. These two species have a greater impact on pepper, causing root necrosis and plant death (14). Southern blight (Athelia rolfsii, sclerotial stage Sclerotium rolfsii) and white mold (Sclerotinia sclerotiorum) can also occur in both crops. White mold is a more serious problem in tomatoes than in pepper, although it occurs in both crops.
Both crops are also affected by root knot nematode (Meloidogyne spp.) (87). Aboveground symptoms of root-knot infection include stunting, wilting, and chlorosis. Gall formation on the roots is the most obvious symptom, particularly on tomato (Fig. 7). Galling on pepper roots may not be as pronounced.
| |

Fig. 7. Tomato roots severely galled as a result of root-knot nematode, Meloidogyne incognita. Complexes of plant pathogenic fungi and root-knot nematode can work synergistically to cause devastating losses to the tomato crop. |
|
Phytophthora blight, caused by Phytophthora capsici is often the most devastating disease impacting pepper. It occurs sporadically throughout Florida and can impact the primary pepper crop as well as the secondary cucurbit crop (103). Infected plants wilt quickly and entire fields can be taken down within a matter of days (Fig. 8). All parts of the plant are susceptible and inoculum can be present in the field, moved with irrigation water, and carried by blowing sand.
Fig. 8. Phytophthora blight epidemics in pepper production in solarized plots (left) and
conventional plots (treated with methyl bromide) (right), following hurricane Irene in 1999.
Weeds are also extremely important in tomato and pepper and represent some of the most difficult pests to control. Nutsedges are consistently problematic throughout Florida (Fig. 9). These weeds can occur in vast monocultures and have a severe impact on crop yields (81,108).
| |

Fig. 9. Purple nutsedge (Cyperus rotundus) growth early in a tomato cropping cycle. |
|
In addition to nutsedges, pigweeds (Amaranthus spp.), nightshades (Solanum spp.), ragweed (Ambrosia artemisifolia) and eclipta (Eclipta prostrata) are common problems. The weed pests in these crops represent a two-fold problem in many cases. In addition to causing significant crop losses due to direct and indirect competition with crop plants, several of these weeds serve as hosts to a number of pathogens that affect the crops in which they are found. Solanaceous weeds harbor several pathogens, including viruses (Fig. 10) that are important in the production of solanaceous crops (1,19,71).
| |

Fig. 10. Nightshade (Solanum americanum) infected with Tomato spotted wilt virus found in a tomato production field. Photo courtesy of S. Adkins. |
|
They are also able to support the reproduction of root-knot nematode, as are the pigweeds (Fig. 11) (92). Nightshade is also reported as a host to Phytophthora capsici (114). Galled nutsedge tubers may also be found in fields with heavy root-knot nematode infestations. Pythium species are commonly isolated from roots of pigweeds, goose grass, and ragweed (Rosskopf, unpublished). Other weeds that are commonly found in production fields include purslane (Portulacca oleracea), which is a host to P. capsici (Ronald French-Monar, University of Florida, personal communication) and numerous grasses that support pathogen as well as nematode populations.
| |

Fig. 11. Pigweed (Amaranthus spp.) will severe galling caused by root-knot nematode (Meloidogyne sp.). |
|
Methyl Bromide-Dependent Crops: Florida Strawberry
Production. Florida currently ranks second to California in strawberry (Fragaria ananassa) production in the U.S., with 15% of the total crop, and 100% of the domestically grown winter crop (36). The majority of Florida's strawberry acreage is located in a concentrated region within a 40-km radius of Plant City, FL, on approximately 2,760 ha (2002 estimate) (34). Strawberry is one of the most expensive crops to produce with average costs per hectare estimated at $42,750 in 1998-99 (66). The average farm is comprised of approximately 16 ha and most farms are located in, or very near residential areas. Florida strawberry production and crop values are summarized in Table 1.
Table 1. Florida Strawberry Production and Acreage farm gate values*
Crop
Year |
Tota
hectares |
Total
Flats
Produced
(x 1,000) |
Yield/ha
(5.4 kg
flat) |
Value
/flat |
Value
/ha |
Total
Value
(Millions) |
| 2001-02 |
2,760 |
14,667 |
5,315 |
$10.46 |
$55,595 |
$153.5 |
| 2000-01 |
2,600 |
14,083 |
5,417 |
$11.88 |
$64,360 |
$167.3 |
| 1999-00 |
2,520 |
18,375 |
7,292 |
$9.12 |
$66,507 |
$167.6 |
| 1998-99 |
2,480 |
15,500 |
6,250 |
$9.72 |
$60,750 |
$150.7 |
| 1997-98 |
2,480 |
13,433 |
5,417 |
$12.00 |
$65,010 |
$161.2 |
| 1996-97 |
2,440 |
14,772 |
6,042 |
$9.91 |
$59,880 |
$146.1 |
| 1995-96 |
2,400 |
13,000 |
5,417 |
$8.66 |
$46,915 |
$112.6 |
| 1994-95 |
2,400 |
14,000 |
5,832 |
$8.47 |
$47,517 |
$118.6 |
*Source: Florida Agricultural Statistics Service Vegetable Summary, 1994-2003.
In Florida, strawberries are grown as an annual crop using raised beds, with two rows of plants/bed (66). Strawberry production practices begin with land preparation in mid-July to mid-August. Soil fumigant is applied and beds are formed in mid-August to mid-September. Bed fumigation with methyl bromide in combination with chloropicrin is applied approximately two weeks prior to transplanting. A single application at an average rate of 159 to 204 kg/ha is injected into the soil during construction of the raised-beds (84). The bed is then immediately covered with plastic mulch. Transplants are primarily bare root and produced in northern latitude nurseries. One drip irrigation line is typically placed in each bed with overhead irrigation used to help establish bare root transplants (27).
Important pests in Florida strawberry production. The most damaging pests in Florida strawberry production include sting nematode (Belonolaimus longicaudatus) (Fig. 12) and nutsedge. Surveys suggest that plant-parasitic nematodes have potential to be a major problem in at least 40% of Florida’s strawberry acreage (J.W. Noling, personal communication). Sting nematode can be extremely damaging to seedlings and transplants, which often undergo a progressive decline and eventually die. Older plants with extensively developed root systems can also be severely affected by sting nematodes (Fig. 12), and are much more susceptible to drought and injury from salt accumulation (26). Symptoms of sting nematode damage are marginal necrosis on the leaves (Fig. 13) and stunted plants in the field (Fig. 14). Currently, there are no sting nematode resistant or tolerant strawberry varieties and no post-plant nematode control options available.
 |
|
 |
|
Fig. 12. Micrograph of sting nematode (Belonolaimus longicaudatus). |
|
Fig. 13. Foliar symptoms of sting nematode damage on strawberry including marginal necrosis and plant stunting. |
| |

Fig. 14. Sting nematode damage in a commercial strawberry field in Florida. |
|
Currently, there are no sting nematode resistant or tolerant strawberry varieties and no post-plant nematode control options available.

Fig. 15. Purple nutsedge in the latter portion of the production of the second crop in a double-cropping scenario. |
|
The most important weeds in Florida strawberry production are nutsedges, Carolina geranium (Geranium carolinianum), cut-leaf evening primrose (Onoethera laciniata), and black medic (Medico spp.) (120). Nutsedges are capable of penetrating plastic mulch, and all weeds remain a season-long problem in row middles and transplant holes. In addition, their survival through the first crop greatly impacts the following crop (Fig. 15). The strawberry production season in Florida lasts six to seven months, during which the density and composition of weed species change. This makes season-long control with herbicides difficult and requires a combination of control measures (112). Unlike in California, hand weeding is an option in Florida, but is not cost effective.
The most important soilborne pathogens in Florida strawberry production are Phytophthora crown rot (Phytophthora citricola and P. cactorum), and anthracnose (Colletotrichum spp.). Alternative chemical control options for these pathogens include preplant treatment with chloropicrin and season-long use of fungicides (116).
Methyl Bromide-Dependent Crops: Florida Floriculture
| |

Fig. 16. Commercial caladium production in the Lake Placid, FL area on muck soil. |
Production. Floriculture is a highly diverse industry encompassing a wide range of ornamental plants. The portion of this industry that uses fumigation as a means to control soilborne diseases is limited to in-ground production. This consists of cut flowers, cut greens, and ornamental bulbs. Florida ranks first in production of cut greens with an estimated value of $83.4 million and second in production of cut flowers with an estimated value of $21.9 million for 2003 (117). Ornamental Caladium tubers produced in Florida have an estimated value of $15 million. This accounts for 95% of world production. Caladiums are produced in central Florida in a region containing muck soil with high organic matter content (Fig. 16) (48). Caladiums are planted from mid-March through mid-April and are harvested from November to March, making the turn-around time between crops minimal.
The majority of caladiums are produced on open ground, on slightly raised beds without plastic mulch, and with or without shade or cover. The common method of fumigant application is a broadcast application of methyl bromide (Fig. 17) made approximately 15 cm deep with shanks under a solid tarp of high density polyethylene (Fig. 18). Formulations of methyl bromide:chloropicrin (98:2 or 90:10) are most commonly used and are applied at 504 kg/ha. It is estimated that 1416 ha are treated annually in Florida for caladium production, using a total of 622,328 kg of methyl bromide (120).
 |
|
 |
|
Fig. 17. Broadcast application of methyl bromide using 12 foot-wide polyethylene tarps. |
|
Fig. 18. Solid tarping following broadcast fumigation for a floriculture crop that requires supplemental light for marketable flower production. |
Supplemental lighting is required for many crops in order to control flowering. Irrigation in many cut flower crops is performed through overhead sprinklers. The use of shade (saran), plastic covering, lights, and sprinklers requires substantial infrastructure, making it necessary for growers to utilize the land on a permanent basis.
Many floriculture growers in Florida use land that is near the Atlantic or gulf coasts because the mild climate is beneficial for growing a wide variety of specialized flowering plants. These coastal areas are typically urbanized and special considerations for pest management must be made by growers in order to coexist in populated regions. The majority of cut flower farms are directly adjacent to housing developments (Fig. 19), limiting the use of alternative fumigants because of restrictions near dwellings and potable water sources.
| |

Fig. 19. Production of Gerbera daisies immediately adjacent to a housing development. The proximity of occupied dwellings will greatly impact the ability to use alternatives to methyl bromide. |
|
| |

Fig. 20. Impact of root-knot nematode and Pythium root rot on Celosia argentea var. cristata (ornamental cockscomb). |
Important pests in Florida floriculture production. The diversity of floriculture crops dependent upon methyl bromide soil fumigation results in a large number of pests that have the potential to limit crop production in the absence of the fumigant. Table 2 provides a listing of the major pests affecting floriculture plants in Florida (120).
Celosia argentea (ornamental cockscomb) is another crop that is highly dependent upon the use of methyl bromide. Celosia is highly susceptible to root-knot nematode (9) and is also impacted by a number of Pythium spp. (Fig. 20).
The range of pests that affect ornamental crops make the search for alternatives to methyl bromide for this industry particularly challenging (109). Each crop, and sometimes each cultivar, may have different sensitivity to a pesticide. Weed problems in ornamental production are highly variable, and few herbicides are registered for use in these crops. In addition to the diversity of the weed populations present, methyl bromide also has been used to transition from one cultivar to the next. Volunteers of the previous crop are considered weed problems and can lower the value of the current crop.
Table 2. Key pests of major ornamentals currently controlled with methyl bromide.
| Crop |
Pests |
| Antirrhinum |
Nematodes: Belanolaimus longicaudatus, Criconomella spp., Dolichodorus heterocephalus, Meloidogyne spp.
Pythium root rot (Pythium irregulare) |
| Caladium |
Nematode: Meloidogyne spp.
Chalky rot (Fusarium sp.), Pythium root rot (Pythium sp.) |
| Calla lily |
Erwinia soft rot (Erwinia carotovora)
Pythium root rot (Pythium spp.) |
| Delphinium |
Sclerotinia stem rot (Sclerotinia spp.) |
| Dianthus |
Fusarium wilt (Fusarium oxysporum fsp. dianthii) |
| Eustoma |
Nematodes: Meloidogyne spp.
Fusarium wilt, root rot, and stem rot (Fusarium oxysporum, F. solani, F. avenaseaum) |
| Freesia |
Fusarium wilt (Fusarium sp.) |
| Gladiolus |
Fusarium wilt (Fusarium oxysporum fsp. gladioli), Stromatinia neck rot (Stromatinia gladioli) |
| Helianthus |
Downy mildew (Plasmopara halstedii) |
| Hypericum |
Nematodes: Meloidogyne spp.
Pythium root rot (Pythium spp.) |
| Iris |
Fusarium wilt (Fusarium oxysporum fsp. iridis) |
| Larkspur |
Sclerotinia stem rot (Sclerotinia sclerotiorum) |
| Liatris spicata |
Sclerotinia stem rot (Sclerotinia scleroiorum) |
| Lilium |
Pythium root rot (Pythium spp.) |
| Matthiola |
Sclerotinia stem rot (Sclerotinia scleroiorum), Xanthomonas leaf spot (Xanthomonas campestris pv. campetris) |
| Ranunculus |
Pythium root rot (Pythium spp.), Xanthomonas leaf spot (Xanthomonas campestris) |
Bulb, tuber,
corm crops |
Bulbs, tubers, corms from previous season |
| All |
Weeds: Numerous species, including: nutsedge (Cyperus spp.), little mallow (Malva parviflora), sow thistle (Sonchus spp.) |
Chemical Alternatives to Methyl Bromide
Currently registered materials. Attempts to identify methyl bromide alternatives for vegetable, strawberry and floriculture production has led to the re-examination of existing soil fumigants such as 1,3-D, methyl isothiocyanate (MITC) generators and chloropicrin (47,68). These compounds represent what is currently available, and they have been evaluated extensively. MITC-generating materials include metam sodium and metam potassium (Vapam and K-Pam, AMVAC Chemical Corp., Newport Beach, CA), as well as dazomet (Basamid). Metam sodium was introduced in 1954 and is considered a Category 3 pesticide. These materials are limited in their applicability as stand alone fumigants as they can be highly variable in efficacy both yearly and by location. The placement of the material must be directed to control specific target pests and combinations with other available materials are effective (21,110,121). Preliminary work in Florida utilized metam sodium (300 liters/ha) and dazomet (440 kg/ha) applied to the bed surface and immediately incorporated, as well as metam sodium applied through a drip line. These treatments were not successful in controlling the targeted pests (67), although treatments utilizing a combination that included pebulate (Tillam) did provide nutsedge control. In subsequent studies that focused on application methods, it was determined that the optimal application for metam sodium involved a pre-bed spray (935 liters/ha) with rotovation to 15-20 cm prior to final bed preparation combined with pebulate at 4.5 kg/ha. This treatment resulted in Fusarium crown rot and nutsedge control that were equivalent to methyl bromide (72). In a more recent study by Gilreath and Santos (53), the combination of metam sodium (320 kg/ha) combined with pebulate (4.5 kg/ha) provided control of nutsedge that was equivalent to methyl bromide (98:2, at 400 kg/ha) at the beginning and at the end of the season. While this combination has been effective in suppressing weeds and some soilborne plant pathogens, yields rarely reach those achieved when methyl bromide is used. In addition, the dependence on pebulate for nutsedge control now significantly limits the usefulness of these materials due to the lapse in registration of the herbicide in 2002 (96).
Chloropicrin is a Class 1 pesticide and is considered to be an excellent fungicide. It has been shown to have some nematicidal activity (52), although it is not as effective as other existing materials, such as 1,3-dichloropropene (1,3-D). However, its contribution for pathogen control is significant, and the majority of studies include chloropicrin as a component in a multi-tactic approach. Where fungal plant pathogens are the primary problem it may be the only material needed. However, in most Florida production scenarios, the pest problems are more complex. Chloropicrin is an important component in the successful use of 1,3-D and may also be used to enhance herbicide efficacy for the control of nutsedge (82).
1, 3-dichloropropene fumigants were introduced by Dow in 1956. Formulations include Telone II (100% 1,3-D), Telone C-17 (73% 1, 3-D and 17% chloropicrin) and Telone C-35 (65% 1,3-D and 35% chloropicrin). In 2001, Telone EC and InLine were registered as drip application formulations of Telone II and Telone C-35 respectively. Initial studies to identify a chemically based alternative using mixtures of 1,3-D and chloropicrin employed existing application procedures (23). In replicated field trials, the fumigant alternatives lacked broad-spectrum weed control, and the procedure was subsequently modified to include an herbicide application (51,67). At the time, workers present in the field during application of 1,3-D were required wear personal protective equipment including gloves, coveralls, and full-face respirators. These requirements were impractical and potentially dangerous for workers during the summer application period in Florida. Research efforts then focused on separating fumigant application from plastic laying operations by broadcast applying 1,3-D ten days in advance of plastic laying using straight shank, forward swept, or para-chisels arranged in staggered rows (50,90). Deep disking prior to fumigation was recommended to achieve deep placement and uniform diffusion of the fumigant through soil. Using this approach average yield loss in large scale field trials was 21.8% when compared to adjacent methyl bromide treated plots (90), and additional concerns emerged regarding fumigant emissions from treated soil.

Fig. 21. Broadcast application of 1,3-dichloropropene using the Yetter deep placement coulter system. |
|
A deep placement coulter system (Avenger, Yetter Manufacturing Co., Colchester, Illinois, USA) was modified to permit injection of 1,3-D into undisturbed soil (Fig. 21) (13). The intact crust layer at the soil surface served as a barrier to slow fumigant emission from the soil. Sealing devices incorporated in the design further minimized movement of the fumigant up through airspace created by the coulter. Eliminating deep disking prior to fumigation reduced application costs, saved time and expanded the application window. Additional field trials expanded the herbicide application program to account for regional diversity in weed communities and added an additional application of chloropicrin during bed formation. Application rates and procedures currently recommended to growers are included in Table 3. Twenty-one large-scale demonstration/validation trials have been conducted on 12 tomato and pepper farms since 2000 (Table 4). Disease control was equivalent (within 5% of adjacent methyl bromide fumigated areas) in 19 trials, inferior in one and superior in one. Soilborne diseases present in the trial were Fusarium wilt and crown rot of tomato and Phytophthora blight of pepper. Repeated applications in the same field over several years did not lead to an increase in soilborne pests. Application costs for the broadcast-based alternative chemical program were $68 per ha higher than the methyl bromide standard based upon price estimates from November 2004 (Table 5). The addition of a third herbicide, oxyflourfen (Goal) raised costs an additional $28.13 per ha. After re-registration in 1999, label changes were implemented that modified the worker protection requirements for 1,3-D-based materials, making them more accessible for use in Florida conditions. Full-face respirators are no longer required, which significantly reduces the potential for heat stress of field workers during application. In addition, the buffer zones required were reduced from 300 ft to 100 ft.
Table 3. Broadcast-based chemical program for fresh market tomato and pepper.
| Procedure |
Chemical |
Rate per ha |
| 1) Cultivate and seal soil surface |
-- |
-- |
| 2) Broadcast fumigant using deep placement coulter system |
1,3-D:Picx (65:35) |
187-221 liters |
| 3) Wait 10 days |
-- |
-- |
4) Broadcast herbicides using directed spray,
incorporate with cultivator, seal soil surface |
Napropamide
Trifluralin |
2.2 kg
0.6 kg |
| 5) Shank inject additional fumigant during preparation of the plastic-mulched beds |
Pic |
156 kgy |
x 1,3-D = 1,3-dichloropropen and Pic = chloropicrin
y Actual amount used by grower will be less because only planting beds are treated with the fumigant
Table 4. Commercial field demonstration/validation trials for broadcast chemical soil disinfestation program.
| Site |
Year |
Crop |
Comparison relative to adjacent
methyl bromide fumigated areas |
| Size (ha) |
Disease |
Weeds |
Nematodes |
Yield |
| 1 |
2000 |
tomato |
4.1 |
equivalentv |
inferiorw |
equivalent |
NDx |
| 2 |
2000 |
tomato |
5.4 |
equivalent |
inferior |
equivalent |
ND |
| 3 |
2000 |
tomato |
3.2 |
equivalent |
equivalent |
equivalent |
ND |
| 4 |
2000 |
pepper |
4.7 |
equivalent |
equivalent |
equivalent |
ND |
| 5 |
2000 |
pepper |
4.8 |
equivalent |
equivalent |
equivalent |
ND |
| 6 |
2000 |
pepper |
1.6 |
equivalent |
equivalent |
equivalent |
ND |
| 7 |
2000 |
pepper |
0.9 |
equivalent |
equivalent |
equivalent |
ND |
| 8 |
2000 |
tomato |
0.8 |
equivalent |
equivalent |
equivalent |
ND |
| 9 |
2000 |
tomato |
4.0 |
equivalent |
equivalent |
equivalent |
ND |
| 1 |
2001 |
tomato |
46.0 |
equivalent |
equivalent |
equivalent |
ND |
| 4 |
2001 |
pepper |
20.0 |
equivalent |
equivalent |
equivalent |
ND |
| 5 |
2001 |
pepper |
32.0 |
equivalent |
equivalent |
equivalent |
ND |
| 7 |
2001 |
pepper |
0.9 |
equivalent |
equivalent |
equivalent |
ND |
| 1 |
2002 |
tomato |
20.0 |
equivalent |
equivalent |
equivalent |
+17% |
| 7 |
2002 |
pepper |
0.9 |
equivalent |
inferior |
euivalent |
+15% |
| 10 |
2002 |
tomato |
1.2 |
equivalent |
equivalent |
equivalent |
-7.5% |
| 1 |
2003 |
tomato |
10.1 |
equivalent |
equivalent |
equivalent |
+18% |
| 11 |
2003 |
pepper |
10.0 |
equivalent |
equivalent |
equivalent |
-11.5% |
| 12 |
2003 |
pepper |
3.6 |
superiory |
equivalent |
equivalent |
+14% |
| 11 |
2004 |
pepper |
13.5 |
inferior |
equivalent |
equivalent |
-7% |
| 1 |
2004 |
tomatoz |
10.1 |
equivalent |
equivalent |
equivalent |
-15% |
Total area treated 197.8 hectares
Total area treated 494.5 acres
vLevels within 5% of adjacent methyl bromide:chloropicrin fumigated area.
wLevels 5% or more above adjacent methyl bromide fumigated area.
xNot determined.
yLevels 5% or more below adjacent methyl bromide fumigated area
zFields were prepared for planting (disked and plastic mulched beds) 4-6 days after broadcast application was made.
Table 5. Projected application costs for alternative soil disinfestation programs.Florida fresh market tomato and pepper production. November 2004 price estimates, 5 ft row centers.
| Methyl bromide standard |
| Chemical |
Cost
per unit |
Application rate per acre |
Cost
per acre |
Cost
per ha |
| broadcast |
bed
application* |
| MeBr:Pic (67:33) |
$2.70/ lbs |
400.00 |
200.00 |
$540.00 |
$1,350.00 |
| Broadcast-based fumigant alternative |
| Chemical |
Cost
per unit |
Application rate per acre |
Cost
per acre |
Cost
per ha |
| broadcast |
bed
application* |
| Devrinol |
$10.60/lbs |
2 lbs |
-- |
$21.20 |
$53.00 |
| Treflan |
$28/gal |
1 pt |
-- |
$3.50 |
$8.75 |
| Chloropicrin |
$2.25/lbs |
140.00 |
70.00 |
$157.50 |
$393.75 |
| Telone C-35 |
$17.50 |
22.00 |
-- |
$385.00 |
$962.50 |
| TOTAL |
$567.20 |
$1,418.00 |
| Broadcast-based with Goal applied to bed surface |
| Chemical |
Cost
per unit |
Application rate per acre |
Cost
per acre |
Cost
per ha |
| broadcast |
bed
application* |
| Devrinol |
$10.60/lbs |
2 lbs |
-- |
$21.20 |
$53.00 |
| Treflan |
$28/gal |
1 pt |
-- |
$3.50 |
$8.75 |
| Goal |
$90/gal |
2 pt |
1 pt |
$11.25 |
$28.13 |
| Chloropicrin |
$2.25/lbs |
140.00 |
70.00 |
$157.50 |
$393.75 |
| Telone C-35 |
$17.50/gal |
22.00 |
-- |
$385.00 |
$962.50 |
| TOTAL |
$578.45 |
$1,446.13 |
| Under Bed Fumigation |
| Product |
Cost
per unit |
Application rate per acre |
Cost
per acre |
Cost
per ha |
| broadcast |
bed
application* |
VIF**
(white/black) |
$211 per
2400 ft |
--- |
--- |
$766.00 |
$1,915.00 |
| Telone C-35 |
$17.50/gal |
30 GAL |
15 GAL |
$262.00 |
$655.00 |
| HDPE (.75 mil) |
$190 per
6000 ft |
|
|
-$275.00 |
|
| TOTAL |
$753.00 |
$1,882.50 |
*bed application = 50% of area treated
** Note - VIF plastic is replacing HDPE as the plastic mulch
Currently, the best available alternative to methyl bromide for pepper production in Florida is a combination of Telone C-35 (1,3-D + 35% chloropicrin) at 187-221 l/ha broadcast 3-5 weeks before planting, followed by chloropicrin at 78-116 kg/ha shanked into the bed, and a herbicide tank mix of clomazone at 1.1 kg/ha or napropamide (2.24 kg/ha) and s-metolachlor at 1.1 kg/ha over the bed top at plastic laying (13,91). The approach for tomato is much the same (55,91), except for tomato growers in Dade county, where due to Karst topology, Telone-based products cannot be used. In Dade county, the best available alternative is metam sodium (701.5 liters/ha) or metam potassium (561.2 liters/ha) with 160 kg/ha chloropicrin. Additional applications of halosulfuron can be used as a post-emergent directed spray where nutsedge is a problem (120). Vegetable trials focused on enhancing efficacy with Telone-based products, herbicides and the effects of alternatives on the second crop in a double-crop situation are on-going (52, W. M. Stall, University of Florida, personal communication).
The best available alternative for strawberry also consists of Telone C-35 (1,3-dichloropropene + 35% chloropicrin), applied in-bed at 331 liters per treated ha, 3-5 weeks before transplanting. Fumigant application is supplemented by an herbicide tank mix of oxyfluorfen 0.56 kg/ha plus napropamide 4.5 kg/ha. A minimum 30-day interval is required for oxyfluorfen before transplanting. No post-emergent nutsedge material is available at this time for Florida strawberry production (91,120). Although this has been identified as the best available alternative, strawberry growers are still concerned about the increased vegetative growth that is associated with high concentrations of chloropicrin (91).
There has been limited research on the efficacy of alternatives to methyl bromide for floricultural crops in the United States. Although much of the technology and potential alternatives can be transferred from research on vegetables, adequate evaluation of their performance must be completed before informed decisions can be made. The majority of research on alternatives to methyl bromide for floriculture has been conducted in California while few studies have been completed in Florida. Many floriculture products are required by regulatory agencies to be free of nematodes and diseases. Currently, methyl bromide is the only chemical capable of providing a level of control that allows growers to ship products under these guidelines.
In California research has been conducted on several floricultural crops including calla lily (Zantedeschia sp.), Freesia x hybrida, Liatrus spicata, Ranunculus asiaticus, snapdragon (Antirrhinum majus), Delphinium sp., and Gladiolus sp.(28,29,30,31,32,43,44,45,46,106). The alternatives that have been tested include methyl iodide (Midas), sodium azide (SEP-100), 1,3 dichloropropene (Telone C-35, Telone EC, Inline) , chloropicrin, furfural (Multiguard Protect and Multiguard FFA), methyl isothiocyanate generators (including Vapam HL and Basamid), dimethyl disulfide (DMDS), soil solarization, and biofumigation. Several alternatives have been combined to identify synergistic effects. Many of the chemical alternatives have been applied through drip irrigation systems. Many alternatives have soilborne pest control properties, including weeds and fungi, and have potential for use in the floriculture industry. However, there are no published data on the nematicidal properties of these alternatives in cut flower production.
In Florida, research has been conducted on a few floricultural crops including snapdragons (Antirrhinum majus), cockscomb (Celosia argentea), and caladium (Caladium x hortulanum) (20,48,49,76,77,78). Telone and metam sodium based treatments have shown promise in these trials, but additional testing is required to establish a feasible, multi-tactic alternative program.
Unregistered materials. A great deal of research has been conducted evaluating methyl iodide (MI) as a drop-in replacement for methyl bromide. This material was originally developed by researchers in California, where the bulk of initial testing was performed (25). It is an attractive replacement due to its soil mobility and broad-spectrum of activity. It is not associated with ozone depletion and rapidly breaks down when exposed to UV light. Registration of methyl iodide is being sought by Arvesta (San Francisco, CA) under the trade name of Midas®. In California, it has been tested in carrot, peach, cut-flower, and strawberry production systems (109). In south Florida, it has been tested alone and in combination with chloropicrin for control of Phytophthora capsici, root-knot nematode and yellow nutsedge. Control achieved was equivalent to methyl bromide and yields of bell pepper were equivalent to methyl bromide when it was combined with chloropicrin (420 kg/ha and 84 kg/ha MI and pic) (74). Noling and Gilreath (89) conducted microplot trials to evaluate rates of methyl iodide for control of root-knot nematode and yellow nutsedge in tomato. Rates ranged from 28-336 kg/ha. Nutsedge germination and mid-season root-knot nematode counts were significantly reduced by all applications of methyl iodide. Final harvest nematode counts and root gall ratings were not significantly different from the untreated check, which would not allow for a double crop to be planted. Although roots were significantly galled, tomato yields were significantly higher than the untreated check with all rates over 56 kg/ha. Additional studies have been conducted to compare rates for nutsedge and nematode control (24,54). Gilreath and Santos (54) found that the best control of nutsedge was achieved using 392 kg/ha of the 50/50 (MI:pic) formulation.
Propargyl bromide was patented as a soil fumigant by Dow Chemical Co. in 1957. Due to its high volatility, it was taken off of the market after a brief period during which it was used in combination with methyl bromide and chloropicrin. In 1999, a more stable formulation was developed that utilized toluene as a carrier. Albemarle Chemical Company was originally identified as a potential registrant (115). Phytotoxicity resulting from the toluene-formulated product lead to a second formulation. Trials using propargyl bromide were conducted in California and Florida. In Florida, trials evaluating application rates of propargyl bromide ranging from 45-224 kg/ha identified rates between 45-112 kg/ha as effective in controlling all tested pests, including root-knot nematode, Fusarium oxysporum f. sp. lycopersici race 3, Phytophthora capsici (tested using inoculum bags in tomato plots) (Figs. 22 and 23) and yellow nutsedge (93,94). Although the results of all trials conducted with propargyl bromide have been positive, there is no registrant currently identified for this fumigant.
| |
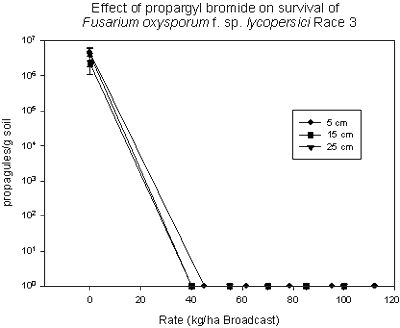
Fig. 22. Impact of increasing rate of propargyl bromide on survival of Fusarium oxysporum inoculum buried at multiple depths. |
|
| |
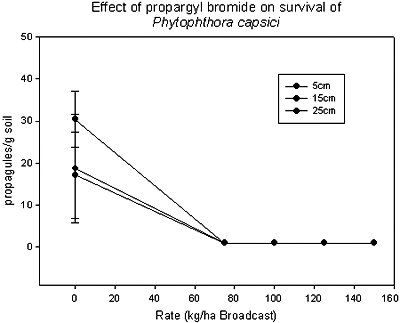
Fig. 23. Impact of increasing rate of propargyl bromide on survival of Phytophthora capsici inoculum buried at multiple depths. |
|
Dimethyl disulfide (DMDS) is currently under development by Cerexagri (King of Prussia, PA), a subsidiary of ATOFINA Chemicals Inc. (Philadelphia, PA), as an alternative to methyl bromide. DMDS has been identified as one of the volatile compounds produced when soil is amended with cabbage and solarized, which leads to a reduction in fungal plant pathogens and nematodes (39). This material has zero ozone depletion potential (ODP) and is reported to have a complex mode of action affecting mitochondrial function and causing inhibition of cytochrome oxidase (4,10). Good control of several soilborne fungal plant pathogens and pathogenic nematodes was achieved in trials in France, Italy and California (37). Recent work with DMDS in cut flowers has produced some encouraging results. Two trials were conducted with growers in Florida, where DMDS was shank applied at 785 kg/ha. At one site, DMDS provided weed control that was equivalent to methyl bromide. Weed pressure at a second site was very low, and no significant differences were detected among any treatments. DMDS provided Pythium root rot control that was comparable to methyl bromide (Fig. 24), and DMDS controlled root-knot nematode (Meloidogyne spp.) juveniles in soil at a level comparable to methyl bromide (Fig. 25). Most importantly, although the DMDS did seem to reduce vegetative growth of cockscomb, marketable yields were equivalent to methyl bromide treatments (Fig. 26) (20).
| |
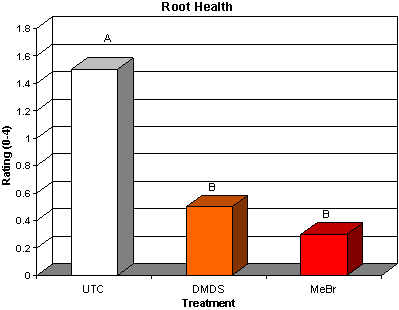
Fig. 24. Root health rating (*scale 0-4: 0=healthy, 1=1-25% root necrosis, 2=26-50% root necrosis, 3=51-75% root necrosis, 4=76-100% root necrosis). Bars with the same letter are not significantly different according to LSD (0.05). Necrotic roots were a result of infection with Pythium spp. |
|
| |
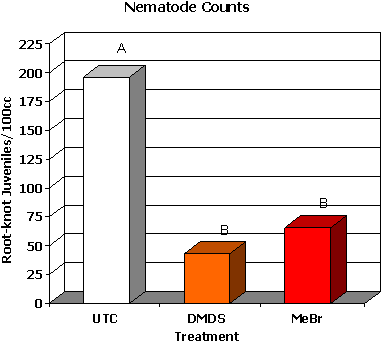
Fig. 25. Mean nematode counts from 100cc composite soils samples collected from each plot. Bars with the same letter are not significantly different according to LSD (0.05). |
|
| |
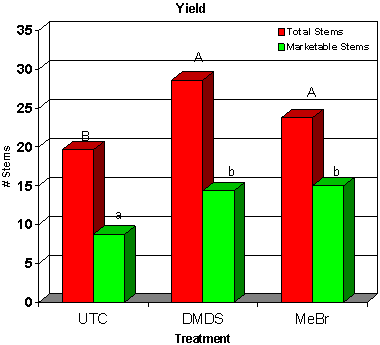
Fig. 26. Plant harvest data. Cuts represents the total number of flowers in each meter of row. Marketable cuts represents the number of these flowers that are saleable according to the grower's standard. Bars with the same letter are not significantly different according to LSD (0.05). |
|
Sodium azide is another material that has been investigated as an alternative to methyl bromide. This material has been reported to have a wide range of activity, including control of nematodes, fungi, and weeds in a variety of crops (104,105). The current formulation sodium azide is referred to as SEP-100™, and it is being registered with the US EPA by American Pacific Corporation. Maximum use rate is 112 kg ai/ha.
Propylene oxide also falls into the near-registration category. This product, which has been used for more than 40 years as a stored-product treatment is currently under development for soil applications by Aberco, Inc. (Seabrook, MD), under the trade name PROPOZONE. It consists of 100% propylene oxide and is shanked or drip applied at rates ranging from 374-935 L/ha. Gilreath et al, (56) found that rates of 748-935 L/ha were required to control nutsedge, but that lower rates were effective in controlling nematodes and fungal plant pathogens. This material is not as effective in cooler climates, but would be an option in Florida. The active ingredient converts to propylene glycol, which is a common food additive.
Many of the previously discussed materials are in the registration process and provide broad-spectrum activity. There are also new chemistries that are currently being investigated for efficacy. A select group of compounds have been screened using laboratory and greenhouse bioassays for their effects on fungi, weeds, and nematodes. Each compound screened falls under all of the three following categories: (i) reduced risk, (ii) biodegradable, and (iii) non-ozone depleting. One compound, referred to as AJMC-330, (Ajay North America, LLC, Powder Springs, GA) has been found to have broad-spectrum activity against multiple fungal plant pathogens (Fig. 27), nutsedge, and root-knot nematode (5,107). Initial field trials with this material have been conducted in Florida tomato and cut-flowers and California strawberries. Florida trials have identified rates and methods that have resulted in effective nutsedge control (Rosskopf, unpublished). Ajwa (H. Ajwa, University of California, personal communication) has conducted trials using AJMC-330 (224 kg/ha) combined with chloropicrin (224 kg/ha) that have resulted in strawberry yields that are 102% of the methyl bromide control (Fig. 28).
| |
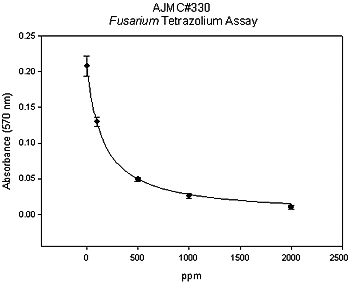
Fig. 27. Reduction in mycelial growth of Fusarium oxysporum f. sp. lycopersici race 3 as a result of exposure to the experimental compound AJMC-330. Growth is measured based on a tetrazolium reaction with living tissue. |
|
| |

Fig. 28. Comparison of the untreated check (left) with the AJMC-330 treatment on the right. Photo courtesy of H. Ajwa. |
|
Virtually Impermeable Films and Under Bed Fumigation
Soil fumigants are broad-spectrum biocides. To ensure that alternative fumigants are used in an effective, economical and environmentally sound manner, soil disinfestation programs must be developed to improve the spectrum of pest control while minimizing negative impacts to the environment.
Application rates can be reduced without comprising efficacy by extending the exposure time of pest propagules to the fumigant (83). This can be accomplished by forcing the fumigant to remain in the soil for an extended period of time. Agricultural films made from polyethylene plastic are highly permeable to soil fumigants and have marginal impact on fumigant retention in soil. (40,99,122,126). Virtually impermeable films (VIF) contain additional polymers that are impermeable to soil fumigants.
To permit the fumigation of existing raised, plastic mulched beds in the absence of drip irrigation systems, a novel apparatus referred to as an ‘under bed fumigator’ was invented to inject fumigants into the soil (12). Fumigation under raised beds that were covered with VIF dramatically improved the retention of 1,3-D and chloropicrin in the soil (Fig. 29). The application potential of a soil disinfestation program that combined the Under Bed Fumigator with VIF (Fig. 30) was validated in six large-scale trials conducted on commercial tomato and pepper farms (Table 6). Application costs were $532 per hectare higher than the methyl bromide industry standard (Table 5). The cost of methyl bromide would have to increase to $3.75/lbs ($1.70/kg) or the price of VIF reduced from $211 per roll to $152 to make the costs equivalent. However, intangible costs such as PPE requirements and liability for workers present in the field at the time of methyl bromide application are eliminated in the Under Bed Fumigation program. The under bed fumigator mitigates regulatory hurdles associated with worker exposure and the use of personal protective equipment by separating the fumigant application from land preparation activities. It also allows growers to make more efficient use of their production fields by creating opportunities to disinfest soil in fields that do not have access to fumigant injection through drip irrigation systems (Fig. 31). A patent application was submitted to the United States Patent & Trademark Office on 3 October, 2002 (Serial No.: 10/263, 107 and Docket No.: 0113.02)
| |
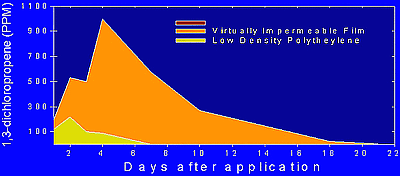
Fig. 29. Retention of the fumigant 1,3-dichloropropene resulting from the use of virtually impermeable film (VIF). |
|
| |
 |
|
 |
|
| |
Fig. 30. Application of 1,3-dichloropropene after the beds are prepared using the Under Bed Fumigator, which limits worker exposure to the fumigant. |
|
Fig. 31. Characteristics and benefits of the Under Bed Fumigation system. |
|
Table 6. Commercial field demonstration/validation trials for soil disinfestation program based upon application of Telone 3-35 into established raised, plastic-mulched beds using the ‘Under Bed Fumigator’ and virtually impermeable films.
| Site |
Year |
Crop |
Comparison relative to adjacent
methyl bromide fumigated areas |
Size
(ha) |
Disease |
Weeds |
Nematodes |
Yield |
| 12 |
2003 |
pepper |
0.4 |
superiorw |
inferiorx |
equivalenty |
+3% |
| 13 |
2003 |
tomato |
1.0 |
superior |
equivalent |
equivalent |
+8% |
| 13 |
2004 |
tomato |
2.0 |
Not determined due to damage from 2 hurricanes |
| 14 |
2004 |
tomato |
2.9 |
Not determined due to damage from 2 hurricanes |
| 15 |
2004 |
tomato |
0.2 |
equivalent |
equivalent |
equivalent |
+7.5% |
| 16 |
2005 |
tomato |
2.5 |
equivalent |
equivalent |
equivalent |
+3.3% |
Total area treated 9.0 hectares
Total area treated 22.5 acres
wLevels 5% or more below adjacent methyl bromide fumigated area
xLevels 5% or more above adjacent methyl bromide fumigated area.
yLevels within 5% of adjacent methyl bromide:chloropicrin fumigated area
zNot determined
Non-Chemical Alternatives
Soil solarization for fresh market tomato and pepper in Florida. In Florida, soil solarization was initially conducted by covering entire fields (broadcast solarization) with clear or photo-selective polyethylene plastic (15,75,97). Subsequently it was adapted to local production systems by using clear plastic to solarize raised beds (strip solarization) and then painting the plastic white so it could be used as a horticultural mulch (16). Solarization on raised beds achieved higher soil temperatures than broadcast applications, thus improving its efficacy and eliminating any border effects (16). Strip solarization was also found to be effective and economically feasible when compared to soil fumigation with methyl bromide:chloropicrin (16,17).

Fig. 32. Large-scale application of soil solarization. The left block is the clear plastic prior to painting and the right side represents the standard methyl bromide/polyethylene film system. |
|
From 1995 to 1999, 18 large-scale trials (Fig. 32) were conducted on commercial tomato and pepper farms (Table 7). A total of 34.8 ha were treated. Some of the trials included combinations of 1,3-D, Pic and solarization. Compared to adjacent areas fumigated with methyl bromide:chloropicrin, levels of pest control were more erratic. Disease control was superior in two trials and inferior in two. Weed and nematode control were inferior in four and five trials, respectively. Compared to adjacent methyl bromide fumigated areas, the average yield was 5% less under soil solarization. The field trials identified variability in the spectrum of pests controlled that was not evident in small plot trials and identified the relationship between pest damage and marketable yields. Technical problems not evident in smaller research plots were also identified including the need to bury drip irrigation tubing in the soil to prevent melting and problems with painting clear plastic using a white latex paint (16,17). Following the large-scale trials, soil solarization was used by a commercial pepper farm to transition from conventional to a biorational farm management system (124).
Table 7. Commercial field demonstration/validation trials for soil solarization program.
| Site |
Year |
Crop |
Comparison relative to adjacent
methyl bromide fumigated areas |
| ha |
Fumigant |
Disease |
Weeds |
Nem-
atodes |
Yield |
| 1 |
1995 |
tomato |
0.4 |
---- |
equivalentv |
equivalent |
inferiorw |
-15% |
| 1 |
1995 |
tomato |
0.4 |
1,3-D+Pic |
equivalent |
equivalent |
equivalent |
-12% |
| 2 |
1995 |
tomato |
0.5 |
---- |
superiorx |
equivalent |
inferior |
7% |
| 3 |
1995 |
tomato |
0.4 |
---- |
equivalent |
equivalent |
inferior |
-1% |
| 4 |
1996 |
tomato |
0.3 |
---- |
equivalent |
inferior |
equivalent |
NDy |
| 5 |
1996 |
tomato |
0.8 |
---- |
equivalent |
inferior |
equivalent |
NDy |
| 5 |
1996 |
tomato |
0.2 |
1,3-D+Pic |
equivalent |
equivalent |
equivalent |
-9% |
| 6 |
1996 |
tomato |
0.6 |
--- |
equivalent |
equivalent |
equivalent |
-5% |
| 6 |
1996 |
tomato |
1.0 |
1,3-D+Pic |
equivalent |
equivalent |
equivalent |
-19% |
| 7 |
1996 |
pepper |
1.3 |
--- |
equivalent |
equivalent |
equivalent |
-7% |
| 7z |
1997 |
pepper |
6.3 |
--- |
equivalent |
equivalent |
equivalent |
11% |
| 8 |
1997 |
tomato |
1.0 |
--- |
equivalent |
inferior |
equivalent |
-8% |
| 7 |
1997 |
pepper |
3.5 |
--- |
superior |
equivalent |
equivalent |
+9% |
| 9 |
1998 |
pepper |
1.2 |
--- |
equivalent |
equivalent |
inferior |
-1% |
| 10 |
1998 |
tomato |
1.1 |
--- |
equivalent |
equivalent |
equivalent |
-4% |
| 7 |
1999 |
pepper |
13.6 |
--- |
equivalent |
equivalent |
equivalent |
ND |
| 11 |
1999 |
tomato |
1.1 |
1,3-D |
inferior |
inferior |
equivalent |
-18% |
| 11 |
1999 |
tomato |
1.1 |
1,3-D+Pic |
inferior |
inferior |
equivalent |
-6% |
Total area treated 34.8 hectares AVG= -5%
Total area treated 87 acres
vLevels within 5% of adjacent methyl bromide:chloropicrin fumigated area.
wLevels 5% or more above adjacent methyl bromide fumigated area.
xLevels 5% or more below adjacent methyl bromide fumigated area
yNot determined
zSolarization repeated in same fields at Site 1996-1999.
Use of biological agents to enhance disease resistance and increase crop vigor and yield. Plant growth-promoting rhizobacteria (PGPR) are beneficial soil bacteria that colonize plant roots and increase plant growth or protect against disease (60). The introduction of PGPR into peat-based growing mixes for vegetable production is an alternative to seed treatment for small-seeded, transplanted crops (38,85). This approach also enables application of larger quantities of beneficial organisms to the root zone of developing plants before transplanting into the field. In order to integrate the application of PGPR into production systems, root colonization under agricultural field conditions was determined and dose-response data for establishment of populations in the rhizosphere were developed (63). A formulation of B. subtilis (strain GBO3) and B. amyloliquefaciens (strain IN937a) was studied following application to pepper (Capsicum annuum) transplants (63). Both isolates established stable populations throughout the root system that persisted through the fall growing season in Florida. Additional post-plant applications of the organisms did not increase their colonization in the rhizosphere compared to treatments receiving bacteria only in the potting medium at seeding. Application of PGPR to transplant plugs at seeding has resulted in increased plant vigor (Fig. 33), reduction in root diseases (Fig. 34), and increased yield in a variety of crops (61,62,65).
| |

Fig. 33. Increased growth and plant vigor seen with the application of plant growth promoting rhizobacteria (PGPR). Pepper plants on the right were treated with Bacillus subtilis strain GBO3 and B. amyloliquefaciens (strain IN937a) compared to the untreated check on the left. |
|
| |
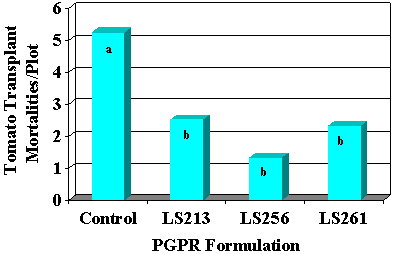
Fig. 34. Survival of tomato transplants in the field was increased significantly with three PGPR formulations tested, as illustrated by a decrease in transplant mortality compared to the control. Treatments with the same letter are not significantly different according to LSD at P < 0.05. |
|
This technology has excellent potential for use in the production of strawberry transplant plugs and in combination with alternative fumigants (62). The ability to improve early yield and reduce transplant shock and water requirements for bare-root transplant establishment may prove advantageous to Florida strawberry producers in the post methyl bromide era. Another beneficial effect of PGPR is the significant increase in transplant growth during greenhouse production, resulting in a shorter production period for standard sized transplants and a reduction in the amount of chemical fertilizers necessary to produce acceptable transplants (64). Because typical disease control levels observed with PGPR treatments are less than those achieved with chemicals, it is most practical to use these organisms as components in integrated management systems that include reduced rates of chemicals, practices to reduce fumigant emissions, and optimum cultural control practices that limit development of pest populations.
There are a substantial number of alternative approaches that have not been covered within the scope of this review. For additional information on research conducted on chemical and non-chemical methyl bromide replacements, particularly in California, Ajwa, Martin and Duniway (2,25,69) provide excellent overviews. While an emphasis is currently being placed on the short-term chemical replacements for methyl bromide, due to the urgency driven by the Protocol, there is a need to be visionary in the development of more sustainable production systems for methyl bromide-dependent crops (11,70). An integrated approach that utilizes biologically-based pest management tactics, such as PGPRs, soil solarization, and biological control agents combined with crop rotations and cover crops will be a necessity in the future. This approach is becoming increasingly important as many agricultural chemicals undergo intense scrutiny with regard to human toxicity and environmental impact during the re-registration process (88). It is uncertain if soil fumigants currently available to growers as methyl bromide alternatives will remain available, or have additional restrictions associated with their use following re-registration (118). It is acknowledged that non-fumigant approaches to crop production for all crops have not been perfected in terms of realizing the broad-spectrum pest control and high yields that have been achieved with methyl bromide. However, it is critical that research in these areas continues to move forward so that the next phase-out does not result in the end of vegetable or ornamental production in the United States.
Mention of a trademark, warranty, proprietary product or vendor does not constitute a guarantee by the United States Department of Agriculture and does not imply its approval to the exclusion of other products or vendors that may also be suitable.
Additional Resources
Additional information on the ozone hole
Stratospheric Ozone: An Electronic Textbook with Low- and High-resolution Graphics and Review Questions
The Ozone Hole Tour by Centre for Atmospheric Science, Cambridge University, UK
Total Ozone Mapping Spectrometer
The Ozone Hole from the The Ozone Hole, Inc.
CEU final ruling Fact Sheet: Final Rule to Create a Critical Use Exemption to the Phase-Out of Methyl Bromide and Exempt Certain Quatities for Critical Uses in 2005Action from the U.S. Environmental Protection Agency
Specific commodity sectors and copies of all U.S. Nominations
Fact Sheet: U.S. Nomination for Methyl Bromide Critical Use Exemptions from the 2007 Phaseout of Methyl Bromide
Protection of Stratospheric Ozone: Process for Exempting Quarantine and Preshipment Applications of Methyl Bromide; Final Rule from the Environmental Protection Agency.
Process for exempting quarantine and preshipment applications Final Rule to Create a Critical Use Exemption to the Phase-Out of Methyl Bromide and Exempt Certain Quatities for Critical Uses in 2005
Comments from stakeholders regarding the allocation of methyl bromide for critical use in Florida Methyl Bromide Critical Use Exemptions -- Today's Concern is Allocation: What About Existing Stocks? from the Florida Fruit & Vegetable Association
Critical Use Exemptions
Conference Proceedings: 2004 Annual International Research Conference on Methyl Bromide Alternatives and Emissions Reductions
Montreal Protocol
Methyl Bromide Critical Use Exemptions -- Today's Concern is Allocation: What About Existing Stocks? from the Florida Fruit & Vegetable Association
U.S. Nomination for Methyl Bromide Critical Use Exemptions from the 2007 Phaseout of Methyl Bromide from the EPA
The Phaseout of Methyl Bromide from the EPA
Literature Cited
1. Adkins, S.A., Markle, L.T., Rosskopf, E.N., and Baker, C. A. 2003. Tomato spotted wilt virus detected in American black nightshade (Solanum americanum) in vegetable field in southeast Florida, Pest Alert.
2. Ajwa, H. A., Klose, S., Nelson, S. D., Minuto, A., Gullino, M. L., Lamberti, F. and Lopez-Aranda, J. M. 2003. Alternatives to methyl bromide in strawberry production in the United States of America and the Mediterranean region. Phytopathol. Mediterr. 42: 220-244.
3. Anonymous. 1998. Montreal Protocol on Substances that Deplete the Ozone Layer. United Nations Environmental Program (UNEP).
4. Auger, J. and Charles, P. 2003. Biogenic emission, biological origin, and mode of action of DMDS, a natural, ubiquitous fumigant. Annual International Research Conference on Methyl Bromide Alternatives and Emissions Reductions, MBAO, p. 138-1.
5. Basinger, W., Rosskopf, E., and Ajwa, H. 2003. Methods of reducing pests by use of halogen substituted ethanol. USDA Patent Application #60/395,230.
6. Blake, N.J., Blake, D.R., Sive, B.C., Chen, T-Y., Rowland, F.S., Collins, Jr., J.E., Sachse, G.W. and Anderson, B.E. 1996. Biomass burning emissions and vertical distribution of atmospheric methyl halides and other reduced carbon gases in the south Atlantic region. Geophysical Research Letters 101:24,151-24,164.
7. Canada CropLife, 2002.
8. Cantliffe, D.J., Hochmuth, G.J., Locascio, S.J., Stansly, P.A., Vavrina, C.S., Polston, J.E., Schuster, D.J., Seal, D.R., Chellemi, D.O., and Olson, S.M. 1995. Production of solanacea for fresh market under field conditions: Current problems and potential solutions. Acta Horticulturae 414:229-244.
9. Cavenesse, F.E. and Wilson, G.F. 1977. Effect of root knot nematodes on growth and development of Celosia argentea L. Acta Horticultura 53:71-73.
10. Charles, P. 2003. DMDS: A new alternative for soil disinfestations. Annual International Research Conference on Methyl Bromide Alternatives and Emissions Reductions, MBAO, pp. 23-1-23-4.
11. Chellemi, D.O. 2002. Nonchemical management of soilborne pests in fresh market vegetable production systems. Phytopathology 92:13671372.
12. Chellemi, D.O., and Mirusso, J. 2004. An apparatus to inject soil fumigants under raised, plastic-mulched beds. Applied Engineering in Agriculture. 20:585-589.
13. Chellemi, D.O., Mirusso, J., Nance, J., and Shuler, K. 2001. Results from field scale demonstration/validation studies on Telone products on the Florida east coast. Proc. Fla. Tomato Institute 2001:51-58.
14. Chellemi, D.O., Mitchell, D.J., Kannwischer-Mitchell, M.E., Rayside, P.A., and Rosskopf, E.N. 2000. Pythium spp. associated with bell pepper production in Florida. Plant Disease 84:1271-1274.
15. Chellemi, D.O., Olson, S.M., and Mitchell, D.J. 1994. Effects of soil solarization and fumigation on survival of soilborne pathogens of tomato in northern Florida. Plant Disease 78:1167-1172.
16. Chellemi, D.O., Oslon, S.M., Mitchell, D.J., Secker, I., and McSorley, R. 1997. Adaptation of soil solarization to the integrated management of soilborne pests of tomato under humid conditions. Phytopathology 87:250-258.
17. Chellemi, D.O. and Rosskopf, E.N. 2004. Yield potential and soil quality under alternative crop production practices for fresh market pepper. Renewable Agriculture and Food Systems 19:168-175.
18. Chipperfield, M.P. and Pyle, J.A. 1998. Model sensitivity studies of Arctic ozone depletion. Journal of Geophysical Research 103: 28,389-28,403.
19. Church, G.T. and Rosskopf, E. N. 2005. First report of the root-knot nematode Meloidogyne arenaria on tropical soda apple (Solanum viarum) in Florida. Plant Disease 89:527.
20. Church, G., Rosskopf, E., and Holzinger, J. 2004. Evaluation of DMDS for production of ornamental cockscomb (Celosia argentea). Proc. Annual Int. Res. Conference on Methyl Bromide Alternatives and Emissions Reductions. MBAO, pp. 87-1-87-5.
21. Csinos, A.S., Webster, T.M., Sumner, D.R., Johnson, A.W., Dowler, C.C., Seebold, K.W. 2002. Application and crop safety parameters for soil fumigants. Crop Protection 21:973-982.
22. Daniel, J.S. Solomon, S., Portmann, R.W., and Garcia, R.R. 1999. Stratospheric ozone destruction: The importance of bromine relative to chlorine. Journal of Geophysical Research 104:23,871-23,880.
23. Dickson, D.W., Locascio, S.J. and Kucharek, T.A. 1995. Evaluation of methyl bromide alternative fumigants and nonfumigants on tomato under polyethylene mulch in 1995. Proc. Annual Int. Res. Conference on Methyl Bromide Alternatives and Emissions Reductions. MBAO, pp.41-1-41-3.
24. Dickson, D.W., Mendes, M. and Hamill, J. 2003. Comparison of methyl iodide formulations with methyl bromide, and an untreated control on the management of root-knot nematodes, weed and yield of tomato in Florida. Proc. Annual Int. Research Conf. on Methyl Bromide Alternative Reductions and Emissions Reductions, MBAO, pp. 22-1-22-4.
25. Duniway, J.M. 2002. Status of chemical alternatives to methyl bromide for pre-plant fumigation of soil. Phytopathology 92:1337-1343.
26. Dunn, R. A., and Noling, J. W. 1997. Florida Nematode Management Guide (SP-54). UF-IFAS, Gainesville, FL.
27. Duval, J.R., Price, J.F., Hochmuth, G.J., Stall, W.M., Olson, S.M., Taylor, T.G., Smith, S.A. and Simonne, E.H. 2005. Strawberry production in Florida. Vegetable Production Handbook for Florida, University of Florida, pp. 277-283.
28. Elmore,C. 1999. Enhancing soil solarization for pest control in field-grown flower crops. Proc. Annual Int. Research Conf. on Methyl Bromide Alternative Reductions and Emissions Reductions, MBAO, p. 48-1.
29. Elmore,C., Roncoroni, J., MacDonald, J., Bolkin, J., Zasala, I., Ferris, H. and Tjosvold, S. 2000. Solarization and biofumigation for field-grown flowers. Proc. Annual Int. Research Conf. on Methyl Bromide Alternative Reductions and Emissions Reductions, MBAO, pp. 96-1-96-3.
30. Elmore,C., Roncoroni, J., Robb, K., Wilen, C. and Ajwa, H. 2001. Preplant pest management in Ranunculus production. Proc. Annual Int. Research Conf. on Methyl Bromide Alternative Reductions and Emissions Reductions, MBAO, pp. 35-1-35-4.
31. Elmore,C., Wilen, C., Robb, K. and Ajwa, H. 2004. Three years of preplant shank, incorporation or drip fumigation in flower crops. Proc. Annual Int. Research Conf. on Methyl Bromide Alternative Reductions and Emissions Reductions, MBAO, p. 32-1.
32. Elmore,C., Roncoroni, J. and Tjosvold, S. 2003. Treatment combinations to improve efficacy in field-grown flowers. Proc. Annual Int. Research Conf. on Methyl Bromide Alternative Reductions and Emissions Reductions, MBAO, pp. 112-1-112-2.
33. Farman, J.C., Gardiner, B.G., and Shanklin, J.D. 1985. Large losses of total ozone in Antarctica reveal seasonal ClOx/NOx interaction. Nature 315:207-210.
34. Florida Agricultural Statistics. 2000. Vegetable Summary. Florida Agricultural Statistics Service, Orlando, FL 32803.
35. Florida Agricultural Statistical Service. 2005. Florida Agriculture Facts.
36. Florida Department of Agriculture and Consumer Services. 1999. Florida Agricultural Facts - 1999 Edition. Tallahassee, FL 32399-0810.
37. Fritsch, J. Baudry, A, and Aubert, T. 2002. Dimethyl disulfide as a new potential alternative to methyl bromide for soil disinfestations. Proceedings of the International Conference on Alternatives to Methyl Bromide, Seville, Spain. P. 340.
38. Gagné, S., Dehbi, L., Le Quéré, D., Cayer, F., Morin, J., Lemay, R. and Fournier, N. 1993. Increase of greenhouse tomato fruit yields by Plant Growth-Promoting Rhizobacteria (PGPR) inoculated into the peat-based growing media. Soil Biol. Biochem. 25: 269-272.
39. Gamliel, A, Austerweil, M., and Kritzman, G. 2000. Non-chemical approach to soilborne pest management-organic amendments. Crop Protection 19:847-853.
40. Gan, J., Yates, S.R., Wang, D. and Ernst, F.F. 1998. Effect of application methods on 1,3-dichloropropene volatilization from soil under controlled conditions. Journal of Environmental Quality, 27:432-438.
41. Geraldson, C.M. 1975. Evaluation of tomato production efficiency with relevance to contributing components. Proc. Fla. State Hort. Soc. 88:152-155.
42. Geraldson, C.M., Overman, A.J., and Jones, J.P. 1965. Combination of high analysis fertilizers, plastic mulch, and fumigation for tomato production on old agricultural land. Soil Crop Sci. Soc. Florida 25:18-24.
43. Gerik,J.S. 2003. Alternative chemical treatments for ornamental crops. Proc. Annual Int. Research Conf. on Methyl Bromide Alternative Reductions and Emissions Reductions, MBAO, pp. 114-1-114-4.
44. Gerik,J.S. 2004. Soil fumigation for Liatris production. Proc. Annual Int. Research Conf. on Methyl Bromide Alternative Reductions and Emissions Reductions, MBAO, pp. 6-1-6-4.
45. Gerik,J.S. 2005. Soil fumigation for Freesia production. Proc. Annual Int. Research Conf. on Methyl Bromide Alternative Reductions and Emissions Reductions, MBAO, pp. 85-1-85-4.
46. Gerik,J.S., Vail, S.S., Elmore, C. and Greene, I.D. 2002. Alternative soil treatments for field grown ornamentals. Proc. Annual Int. Research Conf. on Methyl Bromide Alternative Reductions and Emissions Reductions, MBAO, pp. 93-1-93-3.
47. Gilreath, J., Jones, J., Motis, T., Santos, B., Noling, J. and Rosskopf, E. 2003. Evaluation of various chemical treatments for potential as methyl bromide replacements for disinfestations of soiborne pests in polyethylene-mulched tomato. Proc. Fl. State Hort. Society 116:151-158.
48. Gilreath, J.P., McSorely, R. and McGovern, R.J. 1999. Soil fumigant and herbicide combinations for soilborne pest control in caladium. Proc. Fla. State Hort. Soc. 112:285-290.
49. Gilreath, J.P., McSorely, R. and McGovern, R.J. 2005. Soil fumigant and herbicide combinations for Caladium. Proc. Annual Int. Research Conf. on Methyl Bromide Alternative Reductions and Emissions Reductions, MBAO, pp. 16-1-16-2.
50. Gilreath, J.P., Mirusso, J.M., Jones, J.P., Rosskopf, E.N., Noling, J.W., and Gilreath, P.R. 2002. Efficacy of broadcast Telone C-35 in tomato. Proc. Annual Int. Res. Conference on Methyl Bromide Alternatives and Emissions Reductions. MBAO, pp.19-1-2.
51. Gilreath, J.P., Noling, J.W., Gilreath, P.R., and Jones, J.P. 1997. Field validation of 1,3-dichloropropene + chloropicrin and pebulate as an alternative to methyl bromide in tomato. Proc. Fla. State Hort. Soc. 110:273-276.
52. Gilreath, J.P., Noling, J.W., and Santos, B.M. 2004. Methyl bromide alternatives for bell pepper (Capsicum annuum) and cucumber (Cucumis sativus) rotations. Crop Protection 23:347-351.
53. Gilreath, J.P. and Santos, B.M. 2004. Methyl bromide alternatives for weed and soilborne disease management in tomato (Lycopersicon esculentum). Crop Protection 23:1193-1198.
54. Gilreath, J.P., and Santos, B.M. 2004. Purple nutsedge control with iodomethane. Proc. Annual Int. Research Conf. on Methyl Bromide Alternative Reductions and Emissions Reductions, MBAO, pp. 51-1-51-2.
55. Gilreath, J.P., Santos, B.M., Gilreath, P., Jones, J.P. and Noling, J.W. 2004. Efficacy of 1,3-dichloropropene plus chloropicrin application methods in combination with pebulate and napropamide in tomato. Crop Protection 2004. 23:1187-1191.
56. Gilreath, J.P., Santos, B.M. and Noling, J.W. 2004. Effective rate of propylene oxide for nutsedge control. Proc. Annual Int. Research Conf. on Methyl Bromide Alternative Reductions and Emissions Reductions, MBAO, p. 21-1.
57. Gilreath, J.P. and West, D.W. 1996. Preliminary investigations with fumigant alternatives to methyl bromide in floricultural crops. Proc. FL State Hort. Soc. 112:297-302.
58. Jones, J.P. 1991. Fusarium wilt. Compendium of tomato diseases. APS Press, St. Paul, MN. p. 15.
59. Jones, J.P., Overman, A. J., and Geraldson, C.M. 1966. Effect of fumigants and plastic film on the control of several soil-borne pathogens of tomato. Phytopathology 56:929-932.
60. Kloepper, J. W., Leong, J., Teintze, M. and Schroth, M. N. 1980. Enhanced plant growth by siderophores produced by plant growth-promoting rhizobacteria. Nature 286:885-886.
61. Kloepper, J. W., Reddy, M. S., Kenney, D. S., Kokalis-Burelle, N., Martinez-Ochoa, N. and Vavrina, C. S. 2004a. Theory and application for rhizobacteria in transplant production and yield enhancement. Acta Horticulturae 631: 219-229.
62. Kokalis-Burelle. 2003. Effects of transplant type and soil fumigant on growth and yield of strawberry in Florida. Plant and Soil: 256:273-280.
63. Kokalis-Burelle, N., Kloepper, J. W. and Reddy, M. S. 2005. Population dynamics of Plant Growth-Promoting Rhizobacteria as transplant amendments and their effects on indigenous rhizosphere microorganisms. Applied Soil Ecology: Accepted for publication.
64. Kokalis-Burelle, N., Martinez-Ochoa, N., Rodríguez-Kábana, R. and Kloepper, J. W. 2002b. Development of multi-component transplant mixes for suppression of Meloidogyne incognita on tomato (Lycopersicon esculentum). Journal of Nematology 34: 362-369.
65. Kokalis-Burelle, N., Vavrina, C. S., Rosskopf, E. N. and Shelby, R. A. 2002a. Field evaluation of plant growth promoting rhizobacteria amended transplant mixes and soil solarization for tomato and pepper production in Florida. Plant and Soil 238: 257-266.
66. Legard, D.E., Hochmuth, G.J., Stall, W.M., Duval, J.R., Price, J.F., Taylor, T.G., and Smith, S.A. September 2001. Strawberry Production in Florida. Horticultural Sciences Department Document HS736, University of Florida, Institute for Food and Agricultural Sciences and Florida Cooperative Extension Service.
67. Locascio, S.J., Gilreath, J.P., Dickson, D.W., Kucharek, T.A., Jones, J.P., and Noling, J.W. 1997. Fumigant alternatives to methyl bromide for polyethylene mulched tomato. HortScience 32:1208-1211.
68. Locascio, S.J., Olson, S.M., Gilreath, J.P., Dickson, J.W., Mitchell, D.J., Noling, J.W., Chase, C.A., Sinclair, T.R., and Rosskopf, E.N. 1999. Alternative treatments to methyl bromide for strawberry. Proc. FL State Hort. Soc. 112:297-302.
69. Martin, F. N. 2003. Development of alternative strategies for management of soilborne pathogens currently controlled with methyl bromide. Annu. Rev. Phytopathology 41:325-350.
70. Martin, F.N. and Bull, C.T. 2002. Biological approaches for control of root pathogens of strawberry. Phytopathology 92:1356-1362.
71. McGovern, R.J., Polston, J.E., Mullahey, J.J. 1994. Solanum viarum: weed reservoir of plant viruses in Florida. International Journal of Pest Management 40:270-273.
72. McGovern, R.J., Vavrina, C.S., Noling, J.W., Datnoff, L.A., and Yonce, H.D. 1998. Evaluation of application methods of metam sodium for management of Fusarium crown and root rot in tomato in southwest Florida. Plant Disease 82:919-923.
73. McMillan, Jr., R.T., Bryan, H.H., Ohr, H.D. and Sims, J.J. 1996. Methyl iodide a replacement of methyl bromide as soil fumigant for tomatoes. Proc. Florida State Hort. Society 109:200-201.
74. McMillan, Jr., R.T., Bryan, H.H., Ohr, H.D. and Sims, J.J. 1997. Methyl iodide a direct replacement of methyl bromide as a soil fumigant for sweet peppers. Proc. Annual Int. Research Conf. on Methyl Bromide Alternative Reductions and Emissions Reductions, MBAO, p. 40-1.
75. McSorley, R. and Parrado, J.L. 1986. Application of soil solarization to rockdale soils in a subtropical environment. Nematropica 16:125-140.
76. McSorley, R., and Wang, K.H. 2002. Root-knot nematode problems in flower crops. Proc. Annual Int. Research Conf. on Methyl Bromide Alternative Reductions and Emissions Reductions, MBAO, pp. 3-1-3-2.
77. McSorley, R., Wang, K.H and Church, G. 2004. Comparison of alternative fumigants in cut flower production. Proc. Annual Int. Research Conf. on Methyl Bromide Alternative Reductions and Emissions Reductions, MBAO, pp. 10-1-10-2.
78. McSorley, R., Wang, K.H., Church, G. and Kokalis-Burelle, N. 2004. Evaluation of losses to snapdragon form soilborne pests and diseases. Proc. Annual Int. Research Conf. on Methyl Bromide Alternative Reductions and Emissions Reductions, MBAO, pp. 33-1-33-2.
79. Montzka, S.A., Butler, J.H., Hall, B.D., Mondeel, D.J. and Elkins, J.W. 2003. A decline in tropospheric organic bromide. Geophysical Research Letters 30:ASC 13-1-13-4.
80. Moore, R.M., Webb, M., Tokarczyk, R. and Weaver, R. 1996. Bromoperoxidase and iodoperoxidase enzymes and production of halogenated methanes in marine diatom cultures. Journal of Geophysical Research 101:20,899-20,908.
81. Morales-Payan, J.P., Santos, B.M., Stall, W.M., Bewick, T.A. 1997. Effects of purple nutsedge (Cyperus rotundus) on tomato (Lycopersicon esculentum) and bell pepper (Capsicum annuum) vegetative growth and fruit yield. Weed Technology 11:672-676.
82. Motis, T.N. and Gilreath, J.P. 2002. Stimulation of nutsedge emergence with chloropicrin. Proc. Annual Int. Res. Conference on Methyl Bromide Alternatives and Emissions Reductions. MBAO, pp. 7-1-7-2.
83. Munnecke, D.E. and Van Gundy, S.D. 1979. Movement of fumigant in soil, dosage response and differential effects. Annual Review Phytopathology 17:405-429.
84. National Agricultural Statistical Service, 2002. Census of Agriculture, Pesticide Usage.
85. Nemec, S., Datnoff, L. E. and Strandberg, J. 1996. Efficacy of biocontrol agents in planting mixes to colonize plant roots and control root diseases of vegetables and citrus. Crop Protection 15: 735-742.
86. Newman, P.A., Kawa, S.R. and Nash, E.R. 2004. On the size of the Antarctic ozone hole. Geophysical Research Letters 31:L21104.
87. Noling, J.W. 2002. Nematode Management in Tomatoes, Peppers and Eggplant. UF/IFAS EDIS publication #NG32.
88. Noling, J.W. 2002. The practical realities of alternatives to methyl bromide: Concluding remarks. Phytopathology 92:1373-1375.
89. Noling, J.W. and Gilreath, J.P. 1995. Use of methyl iodide for nematode control. Proc. Annual Int. Research Conf. on Methyl Bromide Alternative Reductions and Emissions Reductions, MBAO, pp. 49-1-49-3.
90. Noling, J.W., and Gilreath, J.P. 2000. Methyl bromide: Progress and problems identifying alternatives. Citrus and Vegetable Magazine. June A3-A14.
91. Noling, J.W. and Gilreath, J.P. 2002. Methy bromide: Progress and Problems Identifying Alternatives, Volume 2. UF-IFAS Extension Bulletin #ENY-49, 18 p.
92. Noling J.W. and Gilreath, J.P. 2002. Weed and nematode management: Simultaneous considerations. Proc. Annual Int. Res. Conference on Methyl Bromide Alternatives and Emissions Reductions. MBAO, p. 6-1-6-3.
93. Noling, J.W., Gilreath, J.P., and Rosskopf, E.N. 2001. Alternatives to methyl bromide field research effort for nematode control in Florida. Proc. Annual Int. Research Conf. on Methyl Bromide Alternative Reductions and Emissions Reductions, MBAO, pp. 14-1-14-3.
94. Noling, J.W., Rosskopf, E.N. and Chellemi, D.O. 2002. Impacts of alternative fumigants on soil pest control and tomato yield. Proc. Annual Int. Research Conf. on Methyl Bromide Alternative Reductions and Emissions Reductions, MBAO, pp. 30-1-30-3.
95. Olson S.M., Maynard, D.M., Hochmuth, G.J., Vavrina, C.S., Stall, W.M., Kucharek, T.A., Webb, S.E., Taylor, T.G., Smith, S.A. and Simonne, E.H. 2005. Tomato production in Florida. Vegetable Production Handbook for Florida, University of Florida, pp. 301-316.
96. Osteen, C. 2003. Methyl bromide phase out proceeds: Users request exemptions. Amber Waves 1:22-27. Accessed April 23, 2005.
97. Overman, A.J. 1985. Off-season land management, soil solarization and fumigation for tomato. Proc. Soil Crop Soc. Fla. 44:35-39.
98. Overman, A.J., Jones, J.P., and Geraldson, C.M. 1965. Relation of nematodes, diseases, and fertility to tomato production on old land. Proc. Florida State Hort. Soc. 78:136-142.
99. Papiernik, S.K., Yates, S. R. and Brown, G.E. 2001. Transport of fumigant compounds through HDPE and virtually impermeable films. Proc. Annual Int. Research Conf. on Methyl Bromide Alternative Reductions and Emissions Reductions, MBAO, pp. 16-1-16-6.
100. Paulus, A.O. 1991. Fusarium wilt. Compendium of tomato diseases. APS Press, St. Paul, MN. p. 14.
101. Redeker, K.R., Wang, N-Y., Low, J.C., McMillan, A., Tyler, S.C. and Cicerone, R.J. 2000. Emissions of methyl halides and methane from rice paddies. Science 290:966-969.
102. Rhew, R.C., Miller, B.R. and Weiss, R.F. 2000. Natural methyl bromide and methyl chloride emissions from coastal salt marshes. Nature 403:292-295.
103. Roberts, P.D., McGovern, R.J., Kucharek, T.A. and Mitchell, D.J. 2001. Vegetable diseases caused by Phytophthora capsici in Florida. UF/IFAS EDIS publication #VH45.
104. Rodriguez-Kabana, R. and Akridge, J.R. 2003. Sodium azide (SEP-100) for control of nematodes and weed problems in green pepper production. Proc. Annual Int. Research Conf. on Methyl Bromide Alternative Reductions and Emissions Reductions, MBAO, pp.46-1-46-8.
105. Rodriguez-Kabana, R., Akridge, J.R. and Burkett, J.E. 2003. Sodium azide (SEP-100) for control of nutsedge, root-knot nematode and Fusarium crown rot in tomato production. Proc. Annual Int. Research Conf. on Methyl Bromide Alternative Reductions and Emissions Reductions, MBAO, pp. 21-1-21-12.
106. Roncoroni, J. and Elmore, C. 2002. Alternatives to methyl bromide in cut flower production in California. Proc. Annual Int. Research Conf. on Methyl Bromide Alternative Reductions and Emissions Reductions, MBAO, pp. 92-1-92-3.
107. Rosskopf, E. and Basinger, W. 2003. Screening of reduced risk compounds for herbicidal and fungicidal properties. Proc. Annual Int. Res. Conference on Methyl Bromide Alternatives and Emissions Reductions, MBAO, pp. 132–1-132-2.
108. Santos, B.M., Bewick, T.A., Stall, W.M., and Shilling, D.G. 1997. Competitive interactions of tomato (Lycopersicon esculentum) and nutsedges (Cyperus spp.). Weed Science 45:229-233.
109. Schneider, S.M., Rosskopf, E.N., Leesch, J.G., Chellemi, D.O., Bull, C.T., and Mazzola, M. 2003. Alternatives to methyl bromide-preplant and postharvest. Pest Management Science 59: 814-826.
110. Seebold, K.W. and Csinos, A. S. 2002. Comparison of application timing on the efficacy of metam sodium and chloropicrin against soilborne fungi. Proc. Annual Int. Res. Conference on Methyl Bromide Alternatives and Emissions Reductions, MBAO, pp. 101-1-101-4.
111. Sonoda, R.M. 1976. The occurrence of a Fusarium root rot of tomato in South Florida. Plant Disease Reporter 60:271-274.
112. Stall, W. M. 1999. Weed control in strawberry. Horticultural Sciences Department Fast Sheet HS-196, UF-IFAS and Florida Cooperative Extension Service.
113. Thomas, W.B., Bedofrd, J.A. and Cicerone, R.J. 1997. Bromine emissions from leaded gasoline. Geophysical Research Letters 24:1371-1374.
114. Tian, D. and Babadoost, M. 2004. Host range of Phytophthora capsici from pumpkin and pathogenicity of isolates. Plant Disease 88:485-489.
115. Trout, T. 2001. USDA program to evaluate propargyl bromide as a soil preplant fumigant. Proc. Annual Int. Research Conf. on Methyl Bromide Alternative Reductions and Emissions Reductions, MBAO, pp. 23-1-23-2.
116. USDA. 2004. Crop Profiles.
117. USDA - National Agricultural Statistics Service Floriculture Crops 2003 Summary.
118. U.S. Environmental Protection Agency, National Agricultural Compliance Assistance Center. 2005.
119. US.-EPA. 2002.The phase-out of methyl bromide.
120. US.-EPA. 2005. U.S. Nomination for Methyl Bromide Critical Use Exemptions from the 2007 Phase-out of Methyl Bromide.
121. Vaculin, P.D. 2004. Metam and chloropicrin combinations as methyl bromide/chloropicrin alternatives. Proc. Annual Int. Res. Conference on Methyl Bromide Alternatives and Emissions Reductions. MBAO, pp. 26-1-26-2.
122. Wang, D., Yates, S.R., Gan, J. and Jury, W.A. 1998. Temperature effect on MeBr volatilization: Permeability of plastic cover films. J. Environ. Quality 26:821-827.
123. Welch, C. 2005. The Ozone Hole.
124. Winsberg, T., Chellemi, D.O., Mellinger, M., and Shuler, K.D. 1998. Transition to a biorational farm management system using soil solarization in a commercial pepper operation. Proc. Fla. State Hort. Soc. 111:78-79.
125. World Meteorological Organization (WMO). 2003. Scientific assessment of ozone depletion: 2002 Global Ozone Research Monitoring Project Report #47, Geneva., Switzerland, 498pp.
126. Yates, S.R., Gan, J., Papiernik, S.K., Dungan, R., and Wang, D. 2002. Reducing fumigant emissions after soil application. Phytopathology 92:1344-1348.
127. Yokouchi, Y., Toom-Sauntry, D., Yazawa, K., Inagaki, T., and Tamaru, T. 2002. Recent decline of methyl bromide in the troposphere. Atmospheric Environment 36:4985-4989.
