Introduction
Citrus Huanglongbing (HLB), better known as citrus greening disease, is a recently introduced disease to the Americas (Brazil and Florida). Numerous reviews on this important disease are available (2,5,6) and include a recent article by Gottwald et al. published jointly in Plant Health Progress and as an APSnet feature article (8). A PDF file has been developed that provides detailed information about the disease and its vectors.
The article by Gottwald et al. addressed the pathogen and its impact, with an emphasis on the history and distribution of the disease, symptoms, the causal organism, its insect vectors, impacts for Brazil and Florida, and potential threats to other citrus producing regions in the Americas. The article extensively dealt with the epidemiology of the disease and the challenges that the temporal increase and spatial spread of the disease have on disease control and management. In this article we will discuss the currently known facts of vector (psyllid) transmission of the citrus greening pathogen and how an understanding of the vector-pathogen interaction affects the current management strategies for HLB.
As previously reviewed by Gottwald et al. (8) HLB is putatively caused by an unculturable bacterium belonging to the α proteobacter. Three types of the disease—Asian, African and American (Brazil)—have been described, with causal bacteria tentatively named Candidatus Liberibacter asiaticus (2), Ca. L. africanus (2) and Ca. L. americanus (17,18,19), respectively. As described, the two known insect vectors of HLB are Diaphorina citri and Trioza erytreae, which inhabit different environmental niches, yet can apparently vector both the Asian or African HLB bacteria. In Brazil D. citri has been found to be the vector of the Ca. L. americanus bacterium (28), limited to Brazil thus far, as well as the Ca. L. asiaticus bacterium. In Florida, HLB is caused by Ca. L. asiaticus, which is vectored by D. citri.
|
|
 Fig. 1. Adult Asian citrus psyllid (Diaphorina citri) feeding on host. Fig. 1. Adult Asian citrus psyllid (Diaphorina citri) feeding on host. |
Biology of the Vector
The Asian citrus psyllid, Diaphorina citri Kuwayama, is a Hemipteran insect measuring 3–4 mm in length with piercing-sucking mouthparts that allow this pest to feed on the phloem of citrus spp. and other related rutaceous plants (13). A summary of known host plants fed on by D. citri is provided by Halbert & Manjunath (9). Adult psyllids are readily observed in aggregations feeding and mating on developing new leaves of host plants (Fig. 1). After mating, female psyllids oviposit (lay eggs) on the new leaf growth of expanding terminals, in the folds of unfurled leaves and behind developing leaf buds. Expanded leaves are not suitable oviposition sites and thus gravid females (females filled with eggs) may migrate in response to scarcity of suitable oviposition sites. A single female psyllid is capable of producing 800 to 1,000 eggs over her lifespan (21).
Eggs are almond shaped and small measuring about 0.3 mm in length thus requiring additional magnification for identification (Fig. 2). Eggs are pale in color when first laid and turn a dark yellow/orange color prior to eclosion (emergence of the first nymphal stage) (13). Eclosion occurs an average of 3 days after the egg is laid but can require more or less time depending on temperature (11).
| |

Fig. 2. Asian citrus psyllid (Diaphorina citri) eggs. |
|
There are five nymphal stages of immature development (Fig. 3). The cumulative duration of the five nymphal stages ranges from 10 to 40 days depending on temperature (11). However, under typical climatic conditions in Florida when a new flush of growth is typically present, the total duration of the nymphal stages is expected to last between 10 to 14 days. Feeding by psyllid nymphs is restricted to the young, tender leaves on which eggs were laid, but also may include the tender portions of the plant branches which have not yet hardened and the succulent stems of developing flowers or newly formed fruit.
| |

Fig. 3. Waxy exudate from Asian citrus psyllid (Diaphorina citri) as it feeds on a leaf. |
|
The two main factors regulating psyllid populations are availability of young growing shoots for oviposition and temperature. Based on these two regulating factors, psyllid populations in Florida citrus groves begin building on the early season growth flushes (Fig. 4) and reach their highest levels on the first summer growth flush, which usually occurs in late May or early June. During the summer months psyllid populations oscillate with the abundance of new flush. During periods of limited availability of unexpanded flush for oviposition, adult psyllids either remain in the plant canopy feeding on mature leaves or migrate to new areas where new flush is available. Summer temperatures above 30°C (86°F) may shorten the longevity of psyllid adults (< 30 days) and lower their reproductive fitness as demonstrated by Liu and Tsai (11). During the slightly cooler (average 26 to 30°C; 79 to 86°F) autumn months in Florida, psyllid populations may increase on the final flushes of the year. Following this flush, adult psyllids will overwinter inside the canopy of citrus plants and are commonly found on the underside of mature leaves feeding on the leaf mid- and lateral veins. At the average daily winter temperature of 15 to 20°C (59 to 68°F) in Florida, adult psyllids can be expected to live on average 50 to 80 days (11) feeding occasionally on their host plants until they move in response to new flush needed for oviposition.
| |

Fig. 4. Blotchy mottle symptoms of citrus greening affected tree with new growth flush showing yellow shoot symptom. |
|
Pathogen Transmission
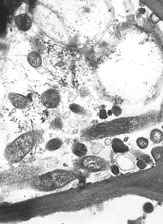
Fig. 5. Electron micrograph of bacteria (Candidatus Liberibacter sp.) in the phloem of infected citrus tree. |
|
Previous studies (26) have shown that adults as well as nymphs have been found to acquire and transmit the causal bacterium. Moll and Martin (14) produced electron microscopy evidence for the presence of bacteria similar to those found in HLB infected citrus (Fig. 5). The reported times for acquisition and transmission of both Asian and African bacteria have varied greatly. For example acquisition times for D. citri have been reported from 15 to 30 minutes (4) and up to 5 h (26). D. citri fourth and fifth instar nymphs can acquire the pathogen and the resulting adults can transmit it (26). Once the bacterium is acquired, the psyllid will retain and transmit the bacterium throughout the psyllid’s life. Some evidence that T. erytreae can acquire and transmit the pathogen transovarially (via eggs) has been published (22).
In Florida it has been found that when D. citri are held for 7 days in the laboratory on HLB infected citrus plants, less than 5% of the psyllids test positive, using a PCR assay, for the presence of Ca. L. asiaticus. However, as confinement time increases on the infected plants, so does the percentage of psyllids testing positive for the presence of Ca. L. asiaticus, with an average percent infection rate between 20% and 30% after a 30 day confinement (Rogers and Brlansky, unpublished). Additionally, adult psyllids have been shown to be capable of acquiring Ca. L. asiaticus from citrus that is infected with the pathogen yet not showing symptoms. For those asymptomatic plants, psyllids were only able to acquire the pathogen from branches of the tree whose leaves gave a positive PCR result when tested, suggesting that due to the uneven distribution of the pathogen within a plant, not all parts of the tree will serve as an inoculum source at any given time (Rogers and Brlansky, unpublished).
Past Approaches to Insect Vectored Disease Management
Insecticide applications to reduce insect populations normally are used to prevent a pest from causing economic damage to a crop. Most of the available information on the use of insecticides for the control of insect vectors of systemic pathogens is for plant virus vectors, especially aphids. Control of insect virus vectors to prevent infection is difficult since only a few winged individuals are necessary to cause considerable spread of the virus. This fact also may be the situation for HLB. Contact insecticides are normally thought to be of limited use since frequent applications are necessary. Persistent insecticides including systemic ones have offered some virus control via vector population control. However, winged aphids (alates) usually carry viruses into crops and may transmit prior to being killed by insecticides. Non-persistent viruses are lost by the aphid upon feeding so insecticides normally do not make any difference in the amount of virus transmission into a crop. However, if aphids that vector a persistent virus are killed, the vectors are halted in their ability to transmit to other plants thus reducing spread. An example of this was shown in potato, where the spread of a persistent virus, potato leaf roll virus (PLRV), was reduced with systemic insecticide applications (3), but a non-persistent potato virus, virus Y, was not (24). Burt et al. (3) pointed out how important reducing the aphid populations early in the season was for successful reduction in virus spread in potatoes. Imidacloprid and three other insecticides were found to reduce the transmission of PLRV by Myzus persicae to potato when exposed to insecticide residues on virus sources (15).
The speed that insecticides kill leafhopper vectors also has been linked to the ability to control viruses that the leafhoppers transmit. Wang et al. (23) suggested that the use of specific systemic insecticides applied when the crops are most susceptible to beet curly top virus (BCTV) could be an effective way to control the curly top disease. The rate of transmission of BCTV, a geminivirus transmitted by the beet leafhopper (Circulifer tenellus) in a circulative, non-propagative manner, was significantly lowered with applications of the systemic insecticide imidacloprid. Soil applications with imidacloprid produced significantly better reduction in BCTV transmission rates than foliar sprays with the insecticide dimethoate. Some success also was reported with the aphid-transmitted sugar beet luteoviruses in England (25) and with barley yellow dwarf luteovirus (12).
Other examples of disease management with insecticides include the control of the whitefly vectors of geminiviruses of vegetable crops. Ahmed et al. (1) found that a systemic insecticide imidacloprid applied two times to control the whitefly, Bemesia tabaci, indirectly controlled the geminivirus tomato yellow leaf curl (TYLCV). However, complete control of virus infection was not obtained; infection rates were lowered early in the growing season, so the tomato crop was protected against disease early in its growth. Imidacloprid is applied to nearly 100% of the tomato acreage in Florida for control of Bemisia argentifolli, the silverleaf whitefly, and the geminiviruses (especially TYLCV) that it transmits (16).
Systemic insecticides also have been used for the control of leafhopper vectors of the xylem-limited, fastidious bacterium Xylella fastidiosa. The glassy-winged sharpshooter, Homalodisca coagulata, a known vector of the various X. Fastidiosa srains that cause Pierce’s disease of grape, phony peach, and citrus variegated chlorosis, was introduced into California in the late 1990s and populations have reached very high levels. Insecticide applications of imidacloprid and other products such as kaolin and harpin have successfully reduced glassy-winged sharpshooter populations in California (20).
Developing New Management Programs
There is general consensus throughout the world literature that three general practices must be adopted in order to have a successful citrus greening management program. These include the planting of certified, clean nursery stock, effective control of psyllid populations and removal of infected trees that serve as an inoculum source for psyllid acquisition. What there is not agreement on, however, is the level of psyllid control needed to be "effective."
Currently, no studies have been conducted proving that managing psyllid populations will indeed provide a benefit in terms of reducing the spread of citrus greening disease. Researchers from countries such as China and South Africa report anecdotal evidence for the need to control psyllids to minimize disease spread and maintain viable citrus production (10,27). However, in each of these growing regions there are differences in climate, cultural practices, and even strains of the pathogen or vector species which makes direct comparison of results difficult.
There are examples where demonstrated low vector populations have limited the spread of the pathogen. In China, for example, greening disease has severely limited citrus production in the lowland areas where D. citri is relatively abundant. In contrast, in the highland areas of China greening disease is not a problem (27). In these areas of higher altitude, D. citri survival is low and thus spread of Ca. L. asiaticus is also low. However, in this particular situation, the cold weather also affects citrus, thus limiting production to mandarin varieties which are better suited for the colder environment.
The situation in Florida is much different as the climate and current production practices are ideal for buildup of large psyllid populations. It is thus impractical to attempt to eliminate psyllids to virtually undetectable levels as this would require an inordinate and unsustainable amount of insecticide applications. A more realistic and sustainable approach to psyllid management would be to target psyllids during the periods when pathogen spread is more likely to occur. This will require knowledge of the seasonal trends for higher percentages of psyllids carrying and transmitting the citrus greening pathogen, if such differences do exist. In addition information is needed regarding when those psyllids are likely moving and infecting healthy trees.
Based on work completed thus far in Florida (discussed above), reducing pathogen spread may be achieved by targeting overwintering adult psyllids. In our research we have demonstrated that the longer psyllids are allowed to feed on infected plant material, the higher the percentage of psyllids that test positive for the presence of the greening pathogen with the highest rates of infection occurring after more than 30 days of feeding. Thus, psyllids that pass the winter months feeding on infected plants are more likely to spread the pathogen to healthy trees when they move in response to the presence of new growth flushes in the spring. Therefore, targeting overwintering adults prior to movement may prove to be an effective approach to minimizing pathogen spread.
There also is evidence that the concentration of the greening pathogen fluctuates within an infected tree throughout the year. PCR diagnostic techniques have been less able to detect the presence of the pathogen (within the same tree) due to the changes in concentration at certain times of the year. Since psyllids do not readily acquire the greening pathogen from sections of an infected plant that do not give a positive Ca. L. asiaticus PCR result, this may mean that psyllid acquisition and movement of the pathogen is also lower at certain times of the year.
Research is underway to determine the seasonality of pathogen transmission by D. citri in Florida and to better define the process of transmission of Ca. L. asiaticus by D. citri. For example, can the use of soil-applied systemic and foliar applied pesticides cause mortality of D. citri carrying Ca. L. asiaticus prior to successful transmission of the pathogen? Based on the results of these research objectives, we may be able to design more effective strategies for psyllid management in order to manage the spread of citrus greening disease and maintain a viable citrus production system.
Additional Resources
From the IFAS Citrus Research and Education Center:
Citrus Greening (Huanglongbing) Disease Identification and Management - PDF format, 1.5 MB
Literature Cited
1. Ahmed, N. E., Kanan, H. O., Sugimoto, Y., Ma, Y. Q., and Inanaga, S. 2001. Effect of imidacloprid on incidence of tomato yellow leaf curl virus. Plant Dis. 85:84-87.
2. Bové, J. M. 2006. Huanglongbing: A destructive, newly-emerging, century-old disease of citrus. J. Plant Pathol. 88:7-37.
3. Burt, P. E., Heathcote, G. D., and Broadbent, L. 1964. The use of insecticides to find when leaf roll and virus Y spread within potato crops. Ann Appl. Biol. 54:13-22.
4. Capoor, S. P., Rao, D. G., and Viswanath, S. M. 1974. Greening disease of citrus in the Deccan trap country and its relationship with the vector, Diaphorina citri Kuwayama. Pages 43-49 in: Proc. of the 6th Conf. of the Intn'l Org. of Citrus Virologists. L. G. Weathers and M. Cohen, eds. Univ. of California, Davis.
5. da Graça, J. V. 1991. Citrus greening disease. Annu. Rev. Phytopathol. 29:109-136.
6. da Graça, J. V., and Korsten, L. 2004. Citrus huanglongbing: Review, present status and future strategies. Pages 229-245 in: Diseases of Fruits and Vegetables, Vol. I. S. A. M. H. Naqvi, ed. Kluwer Academic Press, Dordrecht, The Netherlands.
7. Gatineau, F., Loc, H. T., Tuyen, N. D., Tuan, T. M., Hien, N. T. D., and Truc, N. T. N. 2006. Effects of two insecticide practices on population dynamics of Diaphorina citri and huanglongbing incidence in south Vietnam. Page 110 in: Proc. of the Huanglongbing–Greening Intl. Workshop, July 16-20, 2006, Ribeirão Preto, Brazil.
8. Gottwald, T. R., da Graça, J. V., and Bassanezi, R. B. 2007. Citrus Huanglongbing: The pathogen and its impact. Online. Plant Health Progress doi:10.1094/PHP-2007-0906-01-RV.
9. Halbert, S. E., and Manjunath, K. L. 2004. Asian citrus psyllids (Sternorrhyncha: Psyllidae) and greening disease of citrus: A literature review and assessment of risk in Florida. Fla. Entomol. 87:330-353.
10. Le Roux, H. F., van Vuuren, S. P., Pretorius, M. C., and Buitendag, C. H. 2006. Management of Huanglongbing in South Africa. Pages 43-47 in: Proc. of the Huanglongbing–Greening Intl. Workshop, July 16-20 2006, Ribeirão Preto, Brazil.
11. Liu, Y. H., and Tsai, J. H. 2000. Effects of temperature on biology and life table parameters of the Asian citrus psyllid, Diaphorina citri Kuwayama (Homoptera: Psyllidae). Ann. Appl. Biol. 137:201-206.
12. McKirdy, S. J., and Jones, R. A. C. 1996. Use of imidacloprid and newer generation synthetic pyrethroids to control the spread of barley yellow dwarf luteovirus in cereals. Plant Dis. 80:895-901.
13. Mead, F. W. 1977. The Asiatic citrus psyllid, Diaphorina citri Kuwayama (Homoptera: Psyllidae). Entomol. Circ. No. 180. Fla. Dept. of Agric. and Consumer Serv., Div. of Plant Indust., Gainesville, FL.
14. Moll, J. N., and Martin, M. M. 1973. Electron microscope evidence that citrus psylla (Trioza erytreae) is a vector of greening disease in South Africa. Phytophylactica 5:41-44.
15. Mowry, T. M. 2005. Insecticidal reduction of potato leaf roll virus transmission by Myzus persicae. Ann. Appl. Biol. 146:81-88.
16. Schuster, D. J., Stansly, P. A., Polston, J. E., Gilreath, P. R., and McAvoy, E. 2007. Management of whiteflies, whitefly vectored plant virus, and insecticide resistance for vegetable production in southern Florida. ENY-735, Univ. of Fla., Gainesville, FL.
17. Teixeira, D. C., Ayres, A. J., Kitajima, E. W., Tanaka, F. A. O., Danet, J. L., Jagoueix-Eveillard, S., Saillard, C., and Bové, J. M. 2005. First report of a Huanglongbing-like disease of citrus in São Paulo State, Brazil, and association of a new liberibacter species, "Candidatus Liberibacter americanus", with the disease. Plant Dis. 89:107.
18. Teixeira, D. C., Saillard, C., Jagoueix-Eveillard, S., Danet, J. L., Ayres, A. J., and Bové, J. M. 2005. "Candidatus Liberibacter americanus" associated with citrus huanglongbing (greening disease) in São Paulo State, Brazil. Intl. J. Syst. Evol. Microbiol. 55:1857-1862.
19. Teixeira, D. C., Danet, J. L., Eveillard, S., Martins, E. C., Jesus Junior, W. C., Yamamoto, P. T., Lopes, S. A., Bassanezi, R. B., Ayres, A. J., Saillard, C., and Bové, J. M. 2005. Citrus huanglongbing in São Paulo State, Brazil: PCR detection of the 'Candidatus' Liberibacter species associated with the disease. Mol. Cell. Probes 19:173-179.
20. Tubajika, K. M., Civerolo, E. L., Puterka, G. J., Hashim, J. M., and Luvisi, D. A. 2007. The effects of kaolin, harpin, and imidacloprid on development of Pierce’s disease in grape. Crop Prot. 26:92-99.
21. Tsai, J. H., and Liu, Y. H. 2000. Biology of Diaphorina citri (Homoptera: Psyllidae) on four host plants. J. Econ. Entomol. 93:1721-1725.
22. Van den Berg, M. A., van Vuuren, S. P., and Deacon, V. E. 1992. Studies on greening disease transmission by the citrus psylla, Trioza erytreae (Hemiptera: Triozidae). Israel J. Entomol. 25-26:51-56.
23. Wang, H., Gurusinghe, P. de A., and Falk, B. W. 1999. Systemic insecticides and plant age affect beet curly top virus transmission to selected host plants. Plant Dis. 83:351-355.
24. Webley, D. P., and Stone, L. E. W. 1972. Field experiments on potato aphids and virus spread in South Wales 1966/9. Ann. Appl. Biol. 72:197-203.
25. Westwood, F., Bromilow, R., and Dewar, A. 1997. Controlling virus yellows: How far does your Gaucho go? Br. Sugarbeet Rev. 65:39-42.
26. Xu, C. F., Xia, Y. H., Li, K. B., and Ke, C. 1988. Further study of the transmission of citrus huanglongbing by a psyllid, Diaphorina citri Kuwayama. Pages 243-248 in: Proc. of the 10th Conf. Intl. Organ. Citrus Virol. L. W. Timmer, S. M. Garnsey, and L. Navarro, eds. IOCV, Riverside, CA.
27. Xueyuan, Z. 2006. Huanglongbing in China. Page 3 in: Proc. of the Huanglongbing–Greening Intl. Workshop, July 16-20 2006, Ribeirão Preto, Brazil.
28. Yamamoto, P. T., Felippe, M. R., Garbim, L. F., Coelho, J. H. C., Ximenes, N. L., Martins, E. C., Leite, A. P. R., Sousa, M. C., Abrahão, D. P., and Braz, J. D. 2006. Diaphorina citri (Kuwayama) (Hemiptera: Psyllidae): Vector of the bacterium Candidatus Liberibacter americanus. Page 96 in: Proc. of the Huanglongbing-Greening Intl. Workshop, July 16-20, 2006, Ribeirão Preto, Brazil.
