|

Fruit rot (Washington,1998).
Caruso, F.L., Bristow, P.R. and Oudemans, P.V. 2000. Cranberries: The Most Intriguing Native North American Fruit. APSnet Features. Online. doi: 10.1094/APSnetFeature-2000-1100 |
History of Cranberry Cultivation
|

Fig. 1. Pink abnormal branches arising from axillary buds infected with
Exobasidium oxycocci. (Click image for larger view). | |
The American or large-fruited cranberry (Vaccinium macrocarpon Ait.) is indigenous to the North American continent. It can be found along the northern portion of the United States from Maine to Wisconsin, and along the Appalachians to North Carolina. This cranberry is an introduced plant to Oregon, Washington and British Columbia. It is sometimes found with the small-fruited cranberry (Vaccinium oxycoccus L.) in sphagnum bogs within its range. When the first colonists arrived from Europe, they found it growing in peat bogs and marshes and quickly discovered its importance as a food source. Although the native Americans did not cultivate it (called sasemineash by the Narragansett tribe), they gathered berries and used them in pemmican, a mixture of dried meat or fish and berries that was pounded into a pulp, shaped into a cake and dried in the sun. They were the first to make it into a sweetened sauce using maple sugar. The berries were also eaten raw. Cranberries were used as a poultice for wounds and when it was mixed with cornmeal it was an excellent cure for blood poisoning. The juice was used as a dye to brighten the colors of their blankets and rugs. The early settlers called the fruit "craneberry" because before the flower (Fig. 1) expanded, its stem, calyx, and petals resembled the neck, head, and bill of a crane. It may have also come about because cranes found it to be one of their favorite foods. Through usage, the fruit eventually came to be called a cranberry. Historians generally agree that cranberries had to have been on the table for the first Thanksgiving feast.
The first actual cultivation of the cranberry is attributed to Henry Hall, a Revolutionary War veteran who lived in North Dennis, MA on Cape Cod. Hall was the captain of a sailing vessel, and as he passed his property on Cape Cod Bay, he noted how the best producing and most vigorously growing cranberry vines near his home were those that received regular dune sand driven by the northeast winds (today cranberry beds receive a layer of sand every 3-4 years to promote new root and upright growth). He transplanted some of these vines into a fenced in area to protect them from his cattle in 1816. This was an area directly behind his homestead that had been drained and sanded. The vines produced prolific numbers of different types of berries (some he called "Jumbo") and by 1820 he was shipping his cranberries to Boston and New York City. The word spread rapidly and soon many individuals transplanted sods of cranberry vines for their own cranberry "yards" elsewhere on Cape Cod and throughout Massachusetts. The two principal cranberry cultivars still grown in Massachusetts, "Early Black" and "Howes," were selected from the wild in Harwich and Dennis, respectively, on the Cape Cod peninsula in the 1840s. Cranberries were also cultivated in New Jersey in 1835, Wisconsin in 1853, and in Oregon and Washington in the late 1800s.
Initially Boston became the major marketing center for cranberries shipped to markets in the United States and Europe. For such long distance shipping, cranberries were packed in water in barrels that held 100 pounds of the tart fruits. Cranberries in barrels were consumed by the sailors during their voyages to prevent scurvy, in the same way that limes were used by the British sailors. The barrel (100 pounds) became the standard measure of production for cranberries, and is still used today. This measurement is unique to the cranberry. The expansion of the industry exploded in the 1860s after the Reverend Benjamin Eastwood published his book on cranberry cultivation and because prices were high because of the demand during the Civil War. The industry continued to expand with the coming of the railroad. The second important publication on cranberry culture, by J. J. White in 1870, continued to promote expansion of cranberry acreage. By 1900, 21,500 acres were in production. The industry reached its first zenith in 1930-31 when 27,640 acres were harvested. Acreage decreased due to the Great Depression and the onset of false blossom disease in the eastern acreage through the 1940s. The industry reached its low point after the aminotriazole scare of 1959 that occurred when Arthur Fleming, Secretary of the U.S. Department of Health, Education, and Welfare nationally publicized that a certain amount of cranberries were tainted with this herbicide that was a suspected carcinogen. Despite the fact that a minimal amount of acreage (in Oregon) was affected, the damage was done, cranberries had to be destroyed, and the depressed price took much acreage out of production. The industry finally rebounded in the late 1960s and currently there are 50,000 acres worldwide producing cranberries. Within the past decade, considerable new acreage has been planted in Wisconsin, Quebec, Chile and smaller acreage in non-traditional U.S. states and countries in Europe.
The chief cultivars in production are "Ben Lear" (native Wisconsin selection planted in all areas), "Early Black" (Massachusetts and New Jersey), "Howes" (Massachusetts), "McFarlin" (native Massachusetts selection planted in Wisconsin and the Pacific Northwest), "Pilgrim" (hybrid planted in all areas), "Searles" (native Wisconsin selection planted in Wisconsin), and "Stevens" (hybrid planted in all areas).
Cranberries are harvested for processing (juices, sauce, dried fruits other prepared foods) or for fresh fruit. Until the 1940s, cranberries were harvested in unflooded beds with hand-held scoops (Fig. 2) and subsequently with mechanical harvesters (Fig. 3) that combed the berries from the vines. These berries are packaged and stored in the produce section of the supermarket. Approximately 10% of the Massachusetts acreage is now dry-harvested, and a significant acreage is harvested in Washington as well. Water harvesting (Fig. 4) had its origins in Wisconsin in the 1920s, although only a shallow flood was initially utilized. A deeper flood totally covering the vines was adapted in the early 1960s and shortly thereafter, all growing areas began to water harvest the majority of their acreage. This technology can be used because the cranberry fruit consists of four locules that contain significant amounts of air, thus allowing the berries to float. Two misconceptions of cranberry fields are that the plants grow as bushes and that the fields are constantly flooded. Nothing could be further from the truth!
 | |
 |
|
Fig. 2. Harvesting with a hand-held scoop. This mechanical harvesting aid first began to be used about 1900. (Click image for
larger view). | |
Fig. 3. Harvesting "Early Black" in Massachusetts (September, 1982).
(Click image for
larger view). |
|

Fig. 4. A sea of red berries corralled in one corner of a flooded bed (Wisconsin, October 1997).
(Click image for
larger view).
| |
Diseases Affecting Cranberries
Cranberries occupy a unique environment as compared with most other agricultural crops. This native plant thrives under acidic soil conditions with low nitrogen requirements. Most agronomically important plants would die under the optimum growth conditions for cranberry. The spectrum of diseases affecting cranberry is also unique, with several of the fungal pathogens being specialized to cranberry and its relatives. For example, a number of the fruit and leaf infecting fungi are unique to cranberry. Names such as Exobasidium oxycocci, Lophodermium oxycocci, Monilinia oxycocci, Phyllosticta vaccinii, Physalospora vaccinii and Synchytrium vaccinii imply specificity. Notable mycologists such as C. L. Shear, N. E. Stevens, H. F. Bain and more recently M. E. Barr, D. M. Boone, and L. M. Carris have described numerous species from cranberry and other members of the Ericaceae. There are also some notable exceptions of virulent generalists that include cranberry in their extensive host ranges. These include species such as Colletotrichum acutatum, C. gloeosporioides, and Phytophthora cinnamomi.
There are relatively few known diseases of cranberry incited by either bacteria or viruses. One notable exception is the false blossom disease that is caused by a phytoplasma. This disease, originally considered a virus, nearly wiped out cranberry culture in New Jersey and caused major problems in Massachusetts during the 1920s and 1930s. The disease became so severe that large acreages of cranberry beds were abandoned in both states. Approximately 9,000 acres were lost as a result of this disease. It also spawned the USDA breeding program that resulted in the release of several important hybrid cultivars. Since the discovery of the blunt-nosed leafhopper as a vector, insecticide applications have nearly eliminated this disease from commercial plantings. However, it is frequently seen in areas not receiving insecticides, and most recently in germplasm collections.
Nematodes are also not considered major problems for cranberry production. Nematode species such as
Hemicycliophora have been found in high concentrations although no economic damage has been observed.
Cranberry pathology began during the earliest part of the 1900s when C. L. Shear and co-workers at the USDA studied in detail the fungi causing diseases of cranberries in Massachusetts and New Jersey. It was not until 1922 that investigations into diseases of this crop on the Pacific coast were initiated. The USDA sent Henry F. Bain to the Pacific Coast for four consecutive summers to study diseases in Clatsop County, OR.
A distribution of cranberry diseases in North America can be seen in Figure 5.
|
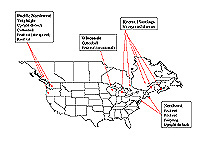
Fig. 5. Distribution of economically important cranberry diseases in North America. (Click image for
larger view).
| |
|

Fig. 6. Twig blight: tan uprights blighted by
Lophodermium oxycocci. (Click image for
larger view).

Fig. 7. Apothecia (open when wet) of
Lophodermium oxycocci on lower surface of a blighted leaf. (Click image for
larger view).
|
Twig blight. This serious vine disease is caused by Lophodermium oxycocci (and possibly L. hypophyllum). While the fungus was originally reported from eastern production areas, the disease has only been of economic importance in Washington and Oregon. The pathogen kills the one-year-old wood on infected uprights. No crop is produced on blighted uprights. Disease incidence can exceed 95% with near complete loss of crop in beds where twig blight is allowed to build up. Ascospores, which are produced from early June through harvest in October, are the only known propagule. Fortunately, infection of new upright growth only takes place during a 5-6 week period during July and early August. Vine symptoms are not observed until late winter or early spring of the following growing season when leaves on the one-year-old wood of infected uprights turn brown. Blighted leaves eventually bleach to a silvery tan (Fig. 6). Fruiting bodies of the fungus (apothecia) develop on the undersides of blighted leaves in spring. These black, football-shaped structures mature in late spring and open along a median slit when wet. The lips of apothecia are gray when open (Fig. 7). Ascospores are forcibly ejected into the air during the morning and early afternoon on days following rain or irrigation. Control is achieved by protecting new upright growth during July and early August with fungicides (2-3 applications; both mancozeb and chlorothalonil are effective). A predictive model based on monitoring the development of ascospores is used to time the first application. These applications reduce disease incidence the following year.
Rose bloom. Three distinct diseases are caused by three different species of
Exobasidium.
Exobasidium oxycocci causes rose bloom, the disease that has the largest impact on crop loss. The fungus infects axillary buds. Each infected bud grows into a pink swollen branch that resembles a miniature rose blossom, hence the common name of this disease (Fig. 8). The fungus sporulates (basidiospores) over the entire surface of these abnormal branches during May and early June (Fig. 9). The noted mycologist A. H. R. Buller pointed out that
Exobasidium spp. induce swelling of host tissues in order to increase the area for spore production and dispersal, thus eliminating the need for an elaborate basidiocarp to maximize the production and dispersal of spores. Basidia erupt from between epidermal cells of the abnormal branch and when basidiospores form, the surface of the branch looks like it has been dusted with powdered sugar. Protectant type fungicides applied when spores are present protect buds on new upright growth from infection and reduce the incidence of the disease the following spring. Infected uprights yield about one-third less than healthy uprights. Loss results from a combination of fewer flowers per flowering upright, lower fruit set and smaller berries. Disease incidence varies from year to year and is very dependent on favorable weather condition for infection.
|
 | |
 | |
|
Fig. 8. Pink abnormal branches arising from axillary buds infected with
Exobasidium oxycocci. (Click image for
larger view).
| |
Fig. 9. Production of basidiospores gives abnormal branch a powdery-white appearance.
(Click image for
larger view).
| |
Cottonball. Ascospores of
Monilinia oxycocci infect the succulent new shoot growth in the spring causing "tip blight." The ascospores are produced in apothecia arising from overwintering psuedosclerotia. Conidia produced on the blighted shoots infect flowers leading to a fruit rot known as "cottonball" or "hard rot" (Fig. 10). Many species of
Vaccinium are attacked by different species of
Monilinia. The species that attacks blueberry (M. vaccinii-corymbosi) causes mummy berry disease and has a disease cycle nearly identical to that of cottonball. Cottonball has only been commercially important in Wisconsin and British Columbia but the disease is also reported from Oregon, Washington, and Nova Scotia. Most of the losses result from the flower infection stage, and consequently, fungicides applied during bloom are the main means of control. Fungicides prevent conidia that land on the stigma (either from wind or insect deposition) from germinating, growing down the style and colonizing the ovary. Once in the ovary, a white cottony mass is formed in the locules (Fig. 11). Infected berries remain symptomless until the time healthy berries begin to turn red. Infected berries remain a distinctive yellow-green and may have tannish-brown striations. The striations correspond to the locule partitions. Eventually the fungus consumes the pericarp and a black, hard pseudosclerotium forms. Mature pseudosclerotia often float and may be disseminated by floodwater for harvest or later for cold protection.
|
 | |
 | |
|
Fig. 10. Cottonball disease cycle:
Monilinia oxycocci. (courtesy P. McManus, University of Wisconsin-Madison).
(Click image for
larger view).
| |
Fig. 11. Cottony fungal growth of
Monilinia oxycocci in locules of infected berries (center) and Yellow-green color of infected berries with tan striations (surrounding).
(Click image for
larger view).
| |

A.

B.
Fig. 12. (A) Symptoms of cranberry vines with the fairy ring disease. Note the areas of dieback within a ring of vegetative over-growth. (B) Color infrared aerial photograph of a cranberry bed with multiple fairy rings. The vegetative overgrowth can be easily seen as the intense red rings with areas of dieback within. (Click images for
larger view).
| |
Fairy ring. This disease is common in Massachusetts and New Jersey but has not been reported in other production regions. This suggests that the pathogen is not disseminated with vine prunings that are used to establish new plantings although mechanical harvesting is thought to be involved in local disease spread. Harvesters may carry along uprooted vines and adhering soil. The first indication of disease is a small area of weak or dead vines. Such areas expand outward in all directions at the rate of about 40 cm per season. The center of larger spots often revives with healthy plants producing a ring (Fig. 12). Newly-infected uprights first turn yellow to a rust color and can be confused with symptoms of upright dieback or drought stress. The causal agent of the disease was originally determined to be the fungus
Psilocybe agariella, although Koch's Postulates were never confirmed. It was suspected that the fungus produced a mat of mycelium around the fine roots of the cranberry plant that limited the water penetrating to the roots. However, investigations are currently in progress to determine whether another causal agent is involved, since the basidiocarps of
Psilocybe have not been observed for several decades. Control measures integrate cultural, nutrition, and fungicide measures. The soil is kept moist by regular irrigation, potassium and magnesium are applied to rejuvenate stressed vines, and affected areas are drenched with ferbam during the peak of the growing season.
|

Fig. 13. Symptoms of upright dieback. (Click image for
larger view).
|
Upright dieback. There may be several fungi involved as causal agents of this disease.
Phomopsis vaccinii is usually cultured from affected uprights. This fungus also causes the fruit rot called "viscid" rot. Other genera that may be implicated in the disease are
Synchronoblastia,
Fusicoccum,
Colletotrichum,
Gloeosporium,
Pestalotia, and
Aureobasidium. Upright symptoms first appear in the spring as yellow mottling or general yellowing of the leaves. The color changes to orange and eventually the upright turns brown and dies (Fig. 13). Dieback of the tip may occur at any time during the growing season, but disease development is apparently favored by warm temperatures when the vines may be under stress from hot and dry conditions. The disease occurs in all production areas and has reached serious levels in British Columbia, Massachusetts, and New Jersey. Cultural practices that encourage vigorous but not excessive vine growth make the vines more resistant to or tolerant of attack. Providing adequate moisture and cooling vines by sprinkling during hot dry periods can help to lessen infection. One application of chlorothalonil or copper hydroxide at budbreak in the spring and other fungicide applications during bloom are effective in reducing vine injury and fruit rot as well.

Fig. 14. A cranberry bed with vines showing acute symptoms of root rot caused by
Phytophthora cinnamomi. Other
Phytophthora species generally cause less dramatic symptoms. (Click image for larger view).
| |
Phytophthora root and runner rot. Given the high moisture level in cranberry soils it is surprising that a disease caused by
Phytophthora spp. was not identified until 1987. Root rot caused by
P. cinnamomi is the most important disease known to affect roots of cranberry. Infection leads to a weakening and eventually death of vines (Fig. 14). Symptomatic vines are unthrifty and may be brittle (brittleness from the continued use of the root-inhibiting herbicide dichlobenil originally resulted in a misdiagnosis of the problem). Below ground, small fibrous feeder roots are sparse or lacking. Infected runner vines may exhibit an olive-green to grayish brown discoloration under the periderm. With the use of new methods for identifying fungi (ELISA and sequencing of ribosomal DNA), this pathogen is much more widespread in cranberry beds in Massachusetts and New Jersey than previously suspected. The first line of defense is to manage soil water. Excessive irrigation should be avoided and drainage should be improved. Drainage can be enhanced by sanding and installing drains, digging deeper side ditches, etc. Applications of fungicides (mainly metalaxyl) have given mixed results and need to be used with cultural methods in an integrated control program. A survey of
Phytophthora spp. isolated from cranberry roots and runners from the different growing regions found
P. cinnamomi restricted to beds in Massachusetts, New Jersey, and Oregon. Other species recovered included
P. megasperma var. megasperma in Washington and Massachusetts,
P. humicola in Washington and New Jersey, and
P. gonopodyides in Washington, Wisconsin, and New Jersey.
P. cryptogea had previously been isolated in Wisconsin. The status of these species as pathogens is unknown at this time. At least five different species of
Pythium were identified in this survey and their status is also unknown.
Focus on Fruit Rot
The cranberry industry was initiated during the nineteenth century by entrepreneurs in the northeast who were interested in shipping loads of this valuable fruit back to Europe where it could be sold for a profit. Unfortunately, soon after methods were established to produce a crop (as opposed to collecting berries from natural populations), cranberry fruit rot became one of the most devastating problems facing growers in the late 1800s. This was a time when plant pathology was in its infancy and data implicating fungi or bacteria as disease-causing agents were sparse. There was little information available to growers on the cause or cure of this disorder. Thus, one of the challenges facing the American Cranberry Growers Association (in New Jersey, where fruit rot has traditionally caused its greatest crop loss) was to assess how best to deal with this problem.
Today, losses from fruit rot are greatly diminished. We accept that fruit rot is not a form of divine retaliation against agriculture but a symptom of intensive farming. It is well established that cranberry fruit rot is a complex disease caused by over fifteen different fungal species. The disease is generally divided into two distinct categories: field rot and storage rot. The field rot phase is expressed pre-harvest and constitutes a major component of direct crop loss. Storage rots cause a reduction in the quality and shelf life of fresh, refrigerated fruit. There is overlap among the fungi that cause field and storage fruit rots. However, there are also fungal species unique to each type. In fungicide efficacy trials, the incidence of field rot is not always correlated with the incidence of storage rot. The management practices for the two phases of the disease differ, and fruit destined for the fresh market is typically harvested and handled in a manner that minimizes storage rot.
|

Fig. 15. Blight flower and infected berry (Phyllosticta vaccinii) (Massachusetts, 1982).
(Click image for
larger view).
|
Field rot is a major threat to cranberry production, especially in New Jersey and Massachusetts where, if left uncontrolled, crop losses in excess of 50% may result. The main reason that berries can be water harvested and air-dried in Wisconsin for subsequent packing as fresh fruit is because fruit rot does not cause appreciable problems in that area. In Massachusetts and New Jersey, once berries are flooded, they decay very quickly thereafter, and they cannot be used as fresh fruit. The most effective control measures rely on nonselective, protectant fungicides including ferbam, mancozeb, and chlorothalonil. In a typical commercial setting, three to five fungicide applications are made during the growing season and resultant field rot levels range from less than 1% to 15%. Currently, fungicide applications begin during early bloom (June 1–15 in New Jersey and two weeks later in Massachusetts) and are repeated on a 7–14 day schedule. Field-rotting fungi are believed to infect early in the growing season and remain latent until the fruit begin to ripen in late August. One exception is the fungus
Phyllosticta vaccinii, which causes an early fruit rot as well as a variety of other symptoms including leaf spot and blossom blight (Fig. 15).
The timing of fruit infections that lead to fruit rot shows relatively low variation, considering the number of fungal species in question. In field experiments, it has been demonstrated that fungal infections leading to fruit rot are concentrated around the period immediately following bloom (July). Fungicide applications initiated during early fruit set, which corresponds to late bloom (early July) showed the greatest efficacy. Treatments initiated after this time showed progressively less effect on disease control. These results suggest that infection must occur within a short window of time in order for fruit rot to occur. Infections occurring later appear to have a reduced probability of developing into field rot. However, those infections may ultimately result in storage rot.
Like the production regions in the northeastern United States, fruit rots are also important diseases of the crop on the Pacific coast, especially those that develop on fresh berries that are held in refrigerated storage between harvest and the holiday periods in November and December. Many of the same fungi causing fruit rots there are the same as those in the east. Table 1 illustrates the more common pathogens of fruit over the years.
Table 1. Historical perspective of fungi most commonly recovered from rotten cranberries in the Pacific Northwest.
|
Year(s) |
Investigator(s) |
Fungi most commonly isolated |
|
1 |
2 |
3 |
| 1922-25 | Bain, H. F. |
Fusicoccum putrefaciens |
Phomopsis sp. |
Coleophoma empetri |
| 1958-61 | Eglitis, M., Gould, C. J. and Johnson F. plus * |
F. putre-
faciens |
Botrytis | -- |
| 1983 | Bristow, P. R. and Windom, G. E. |
F. putre-
faciens |
Colletotrichum gloeo-
sporioides |
Allanto-
phomopsis cytosporea |
| 1991-92 | Keates, S. E. |
C. gloeo-
sporioides |
Allanto-
phomopsis cytosporea |
F. putrefaciens |
| 1996 | Bristow, P. R. and Windom, G. E.* |
C. gloeo-
sporioides |
F. putrefaciens | -- |
* unpublished data.
Fungicides registered for the control of fruit rot are listed in Table 2. In planning a fruit rot management program, one should always observe the preharvest intervals as well as recommendations made by a particular handler. The fungicides chlorothalonil and mancozeb provide the best control of cranberry fruit rot. Ferbam and copper-containing compounds tend to be less effective. There is little difference among the different formulations of chlorothalonil and formulation should reflect an individual preference with regards to ease of handling and cost.
Table 2. Fungicides effective for cranberry fruit rot control
|
Fungicide |
Formulations |
Effectiveness |
Phytotoxicity |
Chloro-
thalonil | Bravo, Terranil, and several others | Very effective under high disease pressure | At high temperatures (>90 F) blossom damage can occur. Fruit scarring has been noted |
| Ferbam | Ferbam | Effective | None reported. Can leave a temporary black residue |
| Mancozeb | Dithane, Manzate | Very effective | Reduces development of anthocyanins |
| Copper | Champ, Kocide | Effective under low disease pressure | None reported from cranberry. Can cause scarring on fruit at high rates |
Fungicides useful for cranberry fruit rot control are broad-spectrum materials. These fungicides will damage plants if they can enter the plant cell. However, these materials are formulated such that they do not cross the cuticle and enter the cell. Therefore, mixing pesticides and use of additives should be done with caution because this can alter the characteristics of the formulation and result in phytotoxicity. In particular some of the newer insecticides being registered have additives to enhance uptake. Mixtures with those insecticides and current fungicides (especially chlorothalonil) will result in phytotoxicity.
Future challenges
Due to increased acreage in production (in both North and South America), decreased advertising and marketing (for unknown reasons) and other factors, there is now a large surplus of cranberries from the past two growing seasons in freezers. Consequently most cranberry growers will receive only $10-18 per barrel for their 2000 crop; compared to the $50-85 they were paid as recently as three years ago. In, addition the USDA marketing order was amended so handlers of berries for processing will accept 15% fewer berries this year. Growers are having to dispose of this excess crop in various ways. Leaving the crop unharvested is not an option for at least two reasons: 1) the build up of inoculum of vine and fruit pathogens and 2) the growth of seedling that are usually unproductive. Berries for the fresh market are not affected by the marketing order. It really hurt to grow food and then inexplicably throw the food away. Many cranberry growers are also hurting economically because for many growing areas, the break-even point for the crop is $35 per barrel.
Farmers are always faced with many tough economic decisions but the current downturn of the industry makes those choices especially difficult because this crop has been one of the most stable agricultural commodities for the last 25 years. Moreover, there are certain diseases, such as twig blight in the Pacific Northwest and cottonball in Wisconsin, that must be controlled. In many cases, several pest management strategies have been reduced or eliminated. Fungicide applications and the rates of these applications have been reduced. Certain cultural practices have been curtailed because growers have a smaller work force. For example, the methods described for the improvement of drainage in beds affected by Phytophthora root rot have been eliminated along with applications of metalaxyl, a very expensive fungicide. Areas of dieback have already begun to increase in size in the past 12 months, and as inoculum of the pathogen increases, these areas will only increase further and new areas of dieback will develop. Where root rot is present, the quality of berries (e.g. fruit rot) will also decline over time. Such a vicious cycle means that we plant pathologists must continue to look for new management strategies that are realistic in this new economic climate. During such a crisis, the need for grower education could not be more important. To make sound decisions, information on the etiology and epidemiology of cranberry diseases and how levels of disease increase as each growing season passes are vital.
Herein lies our biggest challenge. Although plant pathologists have studied diseases of cranberry since the days of C. L. Shear in the early 1900s, for many of the "presumed" causal agents, Koch's Postulates have not been confirmed. Many of the basis epidemiological facts are unknown for key pathogens. Fairy ring is a classic example of this quandary, wherein we assume that that causal agent is
Psilocybe, a fungus that has avoided observation for some time. It is quite possible that this fungus is not there to see, and that another fungal pathogen has quietly been doing its dirty work under the veil of a basidiomycete! Once these pathogens are proven as causal agents, more effective and efficient control strategies can be developed for growers.
As we enter the Thanksgiving, Hanukkah, Christmas, New Year holiday season this year, remember that cranberries are truly a North American treasure. Enjoy!
Compendium of Blueberry, Cranberry, and Lingonberry Diseases and Pests, Second Edition
References
Bain, H. F. 1926. Cranberry disease investigations on the Pacific Coast. USDA Department Bulletin No. 34, 29 pages.
Bristow, P. R. and Windom, G. E. 1985. The impact of machine-harvesting and fungicides on rot and physiological breakdown occurring in cold-stored cranberries. Phytopathology 75:1285.
Buller, A. H. R. 1958. Researches on fungi. Vol. II. Hafner Publishing Co., NewYork, 492 pages.
Burrows, F. A. 1976. Cannonballs and Cranberries. William S. Sullwold, Publisher. Taunton, MA, 96 pages.
Eastwood, B. 1856. Complete Manual for the Cultivation of the Cranberry. C.M. Sexton & Company. New York, 120 pages.
Eck, P. 1990. The American Cranberry. Rutgers University Press. New Brunswick, 420 pages.
Eglitis, M., Gould, C. J. and Johnson, F. 1966. Fungi found on Ericaceae in the Pacific Coastal area. Washington State University, Bulletin No. 675, 21 pages.
Keates, S. E. 1993. Endophytic fungi associated with
Vaccinium macrocarpon Ait. (cranberry) in Washington State and the effect of fungicide treatments on their occurrence. Masters Degree thesis, Washington State University, 220 pages.
Lévesque, C. A., O'Gorman, D., Oudemans, P., Zhu, P., Bristow, P., Windom, G., and McManus, P. 1999. Identification of
Phytophthora and
Pythium isolates from cranberry roots by sequencing of ribosomal DNA. Book of Abstracts, North American Cranberry Research and Extension Workers Conference, Sept. 30- Oct. 2, 1999, Long Beach, WA.
Oudemans, P. V., F. L. Caruso and A.W. Stretch. 1998. Cranberry fruit rot: a complex disease in the northeast. Plant Disease 82(11) 1176-1184
Shear, C. L., Stevens, N. L., and Bain, H. F. 1931. Fungous diseases of cultivated cranberry. USDA Tech. Bull. 258, 57 pages.
Vander Kloet, S. P. 1988. The Genus
Vacinium in North America. Agriculture Canada-Research Branch, Ottawa, Publication 1828, 201 pages.
White, J. J. 1870. Cranberry Culture. Orange Judd Company. New York, 129 pages.
