Introduction
Bermudagrasses (Cynodon spp. and interspecific hybrids) are among the most widely cultivated grass species worldwide. They are also probably the most versatile grasses on the planet, being used for a variety of purposes including erosion control, forage, landscapes, golf courses, athletic fields, and other recreational areas (Fig. 1). These warm-season (C4), perennial grasses grow vigorously in nearly all types of soil, exhibit excellent tolerance to heat and drought, and produce a dense, uniform turf that is resistant to traffic and wear (2,23,29).
| |
Fig. 1. Bermudagrasses are incredibly versatile grass species, used for erosion control, forage, landscapes, golf courses, and athletic fields. On golf courses, varieties of bermudagrass are available for use on tees, greens, fairways, and roughs. |
|
Today, most improved turfgrass varieties of bermudagrass are actually hybrids of common bermudagrass (Cynodon dactylon var. dactylon) and African bermudagrass (Cynodon transvaalensis) (23). These hybrids are sterile triploids (2n=27) and do not produce viable seed, and therefore must be propagated vegetatively via sod or sprigs (Fig. 2). Hybrid bermudagrasses are ideal for use as a turfgrass because they combine the fine leaf texture, upright growth habit, and excellent density of C. transvaalensis with the aggressive growth habit and pest tolerance of C. dactylon (2).

Fig. 2. Most turfgrass areas are established with hybrid bermudagrasses, which must be propagated vegetatively by sod or sprigs. Sprigs are sections of tillers, stolons, and rhizomes, which can be created by shredding pieces of sod. The sprigs are then spread uniformly by hand, then "crimped" into the soil. |
|
Compared to other turfgrass species, bermudagrasses exhibit excellent resistance or tolerance to most pest problems. In fact, there are very few fungal diseases that warrant management. One notable exception is a disease called spring dead spot, caused by fungi in the genus Ophiosphaerella. Each year, spring dead spot causes widespread damage to bermudagrass turf in regions where the turf is exposed to freezing temperatures during winter dormancy. The disease is particularly common and destructive throughout much of the United States, Australia, and New Zealand (17).
As the name implies, symptoms of spring dead spot first appear in the spring, when bermudagrass resumes growth from its normal winter dormancy. As the turf ‘greens-up,’ circular patches or rings of turf appear to remain dormant, and eventually collapse to the ground and die (Figs. 3, 4, and 5). The roots, rhizomes, and stolons in affected patches are sparse and necrotic (Figs. 6 and 7). Although bermudagrass is notorious for its aggressive growth habit and ability to spread quickly, recovery from spring dead spot injury is very slow. The turf in affected patches is often completely dead, so recovery occurs primarily by spread of stolons inward into the patch from the outside perimeter (Fig. 8). In severe cases, complete recovery can take the entire growing season.
 |
|
 |
|
Fig. 3. Symptoms of spring dead spot appear in the spring as soon as the bermudagrass begins to "green-up". |
|
Fig. 4. As the bermudagrass resumes growth, circular patches of turf appear to remain dormant. The dormant foliage in affected patches may remain intact for several weeks. |
| |
 |
|
 |
|
| |
Fig. 5. Eventually, the dormant foliage in spring dead spot patches collapses, leaving sunken depressions in the turf stand that may persist for several months. |
|
Fig. 6. Necrosis of roots, rhizomes, and stolons is evident upon the appearance of spring dead spot symptoms. The majority of pathogen activity is thought to occur in the fall, winter, and spring prior to the onset of disease symptoms. |
|
 |
|
 |
|
Fig. 7. A significant reduction in root depth is noted in spring dead spot patches (left) as compared to healthy turf (right) (Photo courtesy E.L. Butler). |
|
Fig. 8. Recovery from spring dead spot injury is very slow, and may take the entire growing season in severe situations. Although some bermudagrass tillers within the patch may survive, most recovery occurs via spread of stolons into the patch from the outer perimeter. |
Although spring dead spot is a severe and persistent problem in the turfgrass industry, relatively little is known about its biology, epidemiology, and management. The slow progress hasn’t been for a lack of effort. Many researchers across the world have devoted their careers, or a significant portion of them, to the study of spring dead spot and its causal agents. Rather, the complexity of the spring dead spot pathosystem has hindered research progress. Many factors interact to facilitate spring dead spot occurrence, including multiple pathogen species, a host that undergoes a dormant state during winter, freezing temperatures, soil types, thatch accumulation, cultivar susceptibility, and cultural management practices.
Historical Perspective
Spring dead spot was first observed in Oklahoma by a golf course superintendent in 1936 (42), during a time in which the game of golf was becoming a popular meeting place for the nouveau riche of America (18). Dead, circular areas were repeatedly observed in bermudagrass swards as they were greening up from dormancy following the harsh, Oklahoma winter months. Symptoms of this springtime disease became so prevalent in bermudagrass lawns, parks, and golf courses that in the 1950s, D. F. Wadsworth and H. C. Young, of the Department of Botany and Plant Pathology, Oklahoma State University, referred to the disease as spring dead spot (42). At this time, the causal agent was unknown, however, they speculated that it was caused by an unidentified Helminthosporium species isolated from rotted roots. With raised awareness, reports of spring dead spot came from Pennsylvania, Arkansas, Missouri, and Nebraska (18). Wadsworth and Young observed that spring dead spot was most prevalent on well-fertilized, high quality bermudagrass turfs, which led to the first investigations of the relationship between fertilization practices and spring dead spot development. Following three years of study, however, the results were inconclusive (18,42).
By the mid-1960s, spring dead spot had become a widespread problem in the suburbs of Atlanta on lawns that were sodded with ‘Tifway’ hybrid bermudagrass. Although the disease had also been observed on bermudagrass golf courses in Georgia, the demands of homeowners provided incentive to develop a spring dead spot research program at the University of Georgia (18). The emphasis of this research was placed on chemical control, which included multiple fungicide applications throughout the growing season. Following three years of research, it was determined that chemical control results were too inconsistent to make definitive recommendations (18). Cultural practices were also evaluated, with the only successful approach resulting from tilling deep into the root zone to stimulate new sod growth. The causal organism was not yet identified at this time. Kozelnicky (18) and others isolated species of Helminthosporium, Pythium, Fusarium, and Curvularia from rotted bermudagrass roots. However, the disease was not reproducible in greenhouse or field inoculations with these fungi.
A similar patch disease of common bermudagrass (Cynodon dactylon), also known as "couch grass," was being investigated in Australia in the 1960s. Ophiobolus herpotrichus, a dark-mycelium producing root-rot fungus was identified by A.M. Smith as the causal organism (43). Subsequently, Smith (33) identified a second root-rot fungus, Leptosphaeria narmari, and determined that it was more prevalent in Australia. In the meantime, a taxonomic review conducted by Walker and Smith determined that the fungus originally identified as O. herpotrichus more closely resembled a Leptosphaeria, and thereby renamed the fungus L. korrae (43). Smith determined the optimum temperature for growth of L. narmari and evaluated fungicides for controlling spring dead spot in couch grass that included mercuric chloride, phenyl mercury acetate, methyl arsine oxide, thiram, and nabam (33). Of these, thiram and nabam provided control throughout the season.
Spring dead spot research in the United States was gaining momentum in the 1980s. Researchers in California and Maryland independently isolated and identified L. korrae as the causal organism of spring dead spot (6,10). Attempts to isolate L. narmari and L. korrae from bermudagrass exhibiting spring dead spot symptoms in Kansas proved futile, until Tisserat et al. (37) isolated and characterized Ophiosphaerella herpotricha, which is synonymous with Ophiobolus herpotrichus, the original name assigned to the spring dead spot pathogen in Australia (43). Gaeumannomyces graminis var. graminis, an ectotrophic root-infecting fungus, was isolated from ‘Tifway’ bermudagrass roots with spring dead spot symptoms in North Carolina. Pathogenicity evaluations confirmed that G. g. graminis induced root and shoot necrosis of bermudagrass in the greenhouse (27), but this pathogen has not been shown to induce spring dead spot symptoms in the field, and is now known to be the cause of a different disease, bermudagrass decline (9).
Shoemaker and Babcock (32) proposed a new combination, changing L. korrae to Ophiosphaerella korrae based on ascospore and ascocarp morphological characteristics. Tisserat et al. (36) supported this reclassification based on the close phylogenetic relationship of L. korrae to Ophiosphaerella. After observing DNA sequence similarities among O. herpotricha, O. korrae, and L. narmari, L. narmari was also reassigned to Ophiosphaerella and confirmed as the third pathogen of spring dead spot in North America (49).
Ophiosphaerella Species, Biology, and Distribution
Members of the genus Ophiosphaerella are Ascomycetes belonging to Order Pleosporales and Family Phaeosphaeriaceae. Ophiosphaerella species are primarily soil-dwelling fungi that produce asci and ascospores within erumpent pseudothecia (Fig. 9). To date, three species of Ophiosphaerella have been documented as causes of spring dead spot: O. korrae, O. herpotricha, and O. narmari. These species were initially distinguished from one another based on characteristics of the pseudothecia, asci, and ascospores (37,43), but differences in colony morphology and growth rate in vitro are also noted. Phylogenetic analysis of ITS-rDNA regions has provided additional support for this taxonomic distinction (36,51).
| |

Fig. 9. Pseudothecium of Ophiosphaerella korrae on a Kentucky bluegrass rhizome (Photo courtesy American Phytopathological Society). |
|
In pure culture, Ophiosphaerella species are slow-growing, sterile fungi. Colonies are initially white (Fig. 10), and species are indistinguishable at this stage. As the colony matures, it gradually darkens, and some differences are noted among species. Mature colonies of O. herpotricha may be light tan, tannish-gray, light orange, or deep brown, with some isolates not producing aerial hyphae, some forming aerial tufts of mycelia limited to the center of the colony, and some forming tufts that may encompass part or all of the entire surface of the culture (Fig. 11). Colonies of O. korrae produce dense, felt-like aerial mycelium and typically range from light gray to dark gray, but may also be white or black. In contrast, O. narmari typically produces sparse aerial mycelium, with colonies that are white or buff colored.
| |
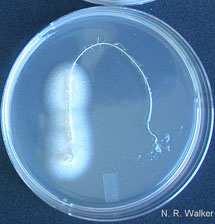
Fig. 10. Ophiosphaerella species are typically isolated by plating symptomatic root or stolon tissue on ¼-strength potato dextrose agar with antibiotics following surface sterilization in 10% bleach for 5 min. The resulting colonies are initially white, and species are indistinguishable at this stage. |
|
| |
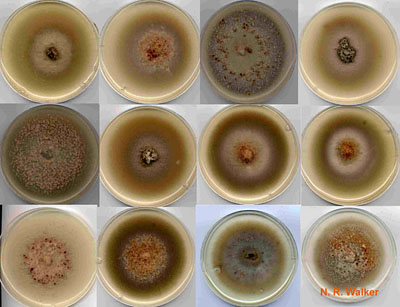
Fig. 11. Comparison of colony morphology among twelve O. herpotricha isolates. Colony morphology is highly variable within and between Ophiosphaerella species, and this is not a reliable characteristic for identification. |
|
Occasional production of pseudothecia by O. korrae and O. narmari has been reported in Australia (33,43). In the United States, pseudothecia have not been observed in nature, but Tisserat et al. (37) observed their formation by O. herpotricha on the stolons and crowns of inoculated bermudagrass plants in the greenhouse. Similar observations have been reported for O. korrae and O. narmari (6,12). The conidial state of O. herpotricha was observed by Webster and Hudson (48) after 6 to 10 weeks of incubation on oat agar, on which pycnidia were produced that were identified as a Scolecosporiella species. No conidial state has been observed for O. korrae or O. narmari. Fertile psuedothecia have not been observed in culture.
Due to the paucity of morphological characters for identification of Ophiosphaerella species, molecular techniques have proven most useful for studies of species distribution. Sauer et al. (31) first developed a species-specific RFLP probe for identification of O. herpotricha. Subsequently, PCR primer sets were developed for selective amplification of the ITS-rDNA regions from each Ophiosphaerella species (36,51). Occasionally, isolates are encountered that appear to be an Ophiosphaerella species but do not react positively to these PCR probes (3). In these cases, sequencing and phylogenetic analysis of the ITS-rDNA region is useful for identification to species.
All three species of Ophiosphaerella have been documented in the United States, but for reasons that remain unknown, they are not uniformly distributed. Ophiosphaerella korrae is the most prevalent cause of spring dead spot in California (10) and the Eastern United States (3,6), whereas O. herpotricha is the primary cause of spring dead spot in the Midwest (16,37,49), extending from North Texas into southern Kansas, as far west as the panhandle of Texas and east into Tennessee (11). Although most US populations are dominated by a single species, O. korrae and O. herpotricha are occasionally isolated from the same location (3,16,33,49), and in some instances, from the same infection center (49). Ophiosphaerella narmari, which is the predominant cause of spring dead spot in Australia and New Zealand (43) is not widely distributed in the United States. This species has been isolated occasionally in California, Oklahoma, Kansas, and North Carolina (16,49,51).
While the complete host range of Ophiosphaerella is unknown, alternative hosts have been identified for O. korrae and O. herpotricha. In addition to causing spring dead spot, O. korrae is also the causal agent of necrotic ring spot, a common disease of bluegrasses (Poa spp.) and fescues (Festuca spp.) in temperate climates (17). Both O. korrae and O. herpotricha induce spring dead spot symptoms in zoysiagrasses (Zoysia spp.) (11,39). In addition, O. herpotricha has been shown to cause spring dead spot in buffalograss (Buchloë dactyloides), which is native to the grasslands of the Midwestern United States (34).
Using AFLP genotyping, Wetzel et al. (49) and Iriarte et al. (16) detected high levels of genetic and genotypic diversity in United States populations of O. herpotricha. In contrast, little diversity was detected in O. korrae or O. narmari populations. The authors suggested that O. herpotricha is native to the United States, where it may have been established in native buffalograss stands, whereas O. korrae and O. narmari may have been recently introduced on bermudagrass introductions or other host plant material.
Pathogenicity and Epidemiology

Fig. 12. Direct infection of the cortical tissue occurs following ectotrophic colonization of the root surface with dark runner hyphae. |
|
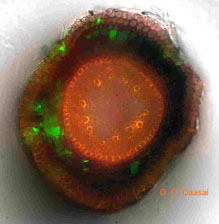
Ophiosphaerella species are referred to as ectotrophic root-infecting fungi, which colonize the surface of below-ground tissues before infecting and causing necrosis. When soil conditions are favorable, Ophiosphaerella species colonize the surface of roots, rhizomes, and stolons with dark runner hyphae, approximately 5 mm in width. During this period, hyphae grow superficially on the root surface and periodically directly infect root cortical tissue (Fig. 12). Infection can occur on roots, stolons or rhizomes, and within days localized necrosis can be observed (Fig. 13). To date, work with transgenetic isolates of O. herpotricha used to follow infection and colonization of roots has not revealed the presence of the fungus inside vascular tissues 14 days after infection (5) (Fig. 14).
 |
|
 |
|
Fig. 13. Necrosis of bermudagrass roots and stolons following infection with Ophiosphaerella herpotricha. |
|
Fig. 14. At Oklahoma State University, transgenetic isolates of O. herpotricha expressing green fluorescent protein (GFP) are being used to monitor the infection process. Here the pathogen can be seen colonizing the root epidermis and cortex, but is not present in the vascular cylinder. |
The optimal temperature for growth of O. korrae and O. herpotricha in vitro is between 20°C (68°F) and 25°C (77°F) (37,43,47), but in the field, the majority of pathogen activity is thought to occur when soil temperatures are less than 21°C (70°F) (6,10,47). These temperatures occur in the fall and spring when the bermudagrass plant is entering or exiting winter dormancy, thus the pathogen has a competitive advantage over the slow-growing host plant during this time. As is the case with take-all patch (13,19), reduced competition from other soil microorganisms may also increase disease activity at lower soil temperatures. Similarly, wet or nearly saturated soils appear to facilitate infection of bermudagrass roots by Ophiosphaerella species (47), and may account for the observation that spring dead spot is more severe in soils with greater water holding capacity (52).
In Oklahoma, infection and colonization of bermudagrass roots by O. herpotricha was found to occur both in spring and autumn months, suggesting that the pathogen has two distinct periods of activity in the Midwestern region (47). Plants remain infected during summer months when warmer temperatures distinctly favor bermudagrass growth (47), but the activity of O. herpotricha inside plants likely decreases or may entirely cease during this time.
In Mississippi, the occurrence of O. korrae on a symptomatic bermudagrass fairway in relation to soil temperature is being investigated. The pathogen has been isolated on a monthly basis from symptomatic bermudagrass roots, but was isolated most frequently during the spring and least frequently during the summer and fall. No correlation between soil temperature and pathogen occurrence was detected. Increased isolation frequency during the spring may be the result of cumulative pathogen activity and infection during the fall, winter, and spring. Vigorous root growth, combined with reduced pathogen activity, would be expected to reduce isolation frequency during the summer and fall.
Early observers and researchers of spring dead spot noted a relationship between freezing temperatures and the severity of the disease (20). Subsequently, research has indicated that freezing temperatures following periods of pathogen activity are required to induce the appearance of symptoms. McCarty et al. (26) and Tisserat et al. (37) demonstrated that bermudagrass infected with the ectotrophic root-rot pathogens did not express spring dead spot symptoms unless they were exposed to freezing soil temperatures. In growth chamber studies, Nus and Shashikumar (30) detected an increase in the susceptibility of bermudagrass plants to cold damage when infected by either O. korrae or O. herpotricha. Also in growth chamber studies, Iriarte et al. (14) induced spring dead spot symptoms in inoculated bermudagrass following brief exposure to freezing temperatures of -2°C (28°F) or lower. Recent field research at Oklahoma State University demonstrates that bermudagrass mortality can occur quickly after entering dormancy or may increase gradually during the course of the winter (45) (Fig. 15). In some cases, symptoms of spring dead spot have been induced when actively growing bermudagrass was exposed to freezing temperatures for short periods of time.
| |
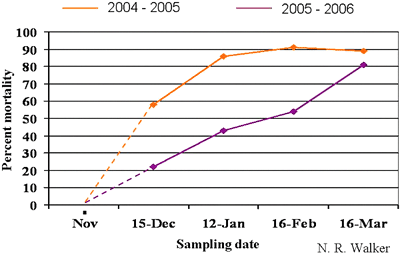
Fig. 15. Mortality of bermudagrass infected with O. herpotricha was monitored by collecting cores of infested turf periodically during the winter and placing in the greenhouse to induce regrowth. In 2004-2005, mortality increased rapidly during December and January and then plateaued. In contrast, mortality increased gradually throughout the sampling period in 2005-2006. |
|
Research has also confirmed that a negative correlation exists between the cold tolerance of bermudagrass cultivars and the expression of spring dead spot symptoms (1,25). The precise mechanisms by which Ophiosphaerella infections reduce the cold-tolerance of bermudagrass have not been investigated, but presumably necrosis of the rhizomes and stolons, the primary over-wintering structures of bermudagrass, reduces the plant’s ability to persist through freezing temperatures.
Ophiosphaerella species have been reported to vary in their virulence toward bermudagrass. In Australia, Smith (33) reported that O. korrae was more virulent than O. narmari, the most widespread spring dead spot pathogen in that country. In greenhouse inoculations of ‘Sahara’ common bermudagrass, Tisserat et al. (37) demonstrated that O. herpotricha caused greater root necrosis and reductions in root weights than O. korrae. However, recent greenhouse inoculations of ‘Sahara’ in Mississippi found that the virulence of O. korrae and O. herpotricha was similar, both causing extensive root necrosis and decline (Fig. 16).
| |

Fig. 16. Necrosis of ‘Sahara’ bermudagrass roots and stolons induced in the growth chamber by inoculation with O. korrae (B) as compared to non-inoculated plants (A). |
|
Iriarte et al. (15) investigated the virulence of Ophiosphaerella species by inoculating field plots of ‘Midlawn’ and ‘Tifway’ bermudagrass in Kansas and Oklahoma. Isolates of O. herpotricha were most virulent, exhibiting the greatest infection efficiency and inducing the largest patches. On the other hand, O. narmari was very weakly virulent in the field, and in some cases, did not induce spring dead spot symptoms. Isolates of O. korrae varied in their virulence toward bermudagrass; some isolates were as virulent as O. herpotricha, whereas others exhibited intermediate virulence.
In North Carolina, Tredway et al. (40) observed similar differences in virulence between O. herpotricha and O. korrae resulting from field inoculations of ‘Tifway’ bermudagrass. A three-isolate mixture of each species was inoculated to 600 individual inoculation points in October 2005. In May 2007, 98% of O. herpotricha inoculations induced spring dead spot symptoms, and patches averaged 22 cm in diameter (Fig. 17). In contrast, only 60% of O. korrae inoculations induced spring dead spot symptoms, and patches averaged 9.4 cm in diameter (Fig. 18). The dominance of O. korrae as a cause of spring dead spot in the Eastern United States is apparently not related to an increased fitness or virulence of this pathogen in this climate. As Iriarte et al. (16) suggested, the distribution of Ophiosphaerella species in the United States may have been determined by the native range of these species and movement on infested plant material.
| |

Fig. 17. Spring dead spot patches resulting from field inoculation of ‘Tifway’ bermudagrass in North Carolina with isolates of O. korrae and O. herpotricha. |
|
| |
 |
|
Fig. 18. As previously found in the Midwestern United States, O. herpotricha is more virulent than O. korrae in North Carolina as well. Nineteen months after inoculation, 98% of O. herpotricha inoculations induced spring dead spot symptoms, and patches averaged 22 cm in diameter (three patches on right), whereas 60% of O. korrae inoculations induced spring dead spot symptoms, and patches averaged 9.4 cm in diameter (three patches on left). |
|
Spring Dead Spot Management
Despite the widespread distribution and destructive nature of spring dead spot, there are few management options available that are effective, economical, and practical. As a result, this disease continues to cause widespread damage to bermudagrass turfs on an annual basis.
Management practices that increase the cold hardiness of bermudagrass generally reduce the incidence of spring dead spot. In fact, use of bermudagrass varieties with improved cold-tolerance is perhaps the most effective and sustainable method for management of this disease (1,25). Spring dead spot is typically more damaging on intensively managed turfs compared to low maintenance areas (22). Late-season applications of nitrogen have been shown to increase disease severity the following spring (20,28). Excessive applications of nitrogen during the summer have also been observed to stimulate disease development, perhaps due to an accumulation of plant available nitrogen in the soil profile (20). Applications of potassium in the fall prior to dormancy have been thought to increase bermudagrass cold tolerance and reduce spring dead spot development (21). However, McCarty et al. (28) found that fall applications of potassium sulfate at high rates (269 kg/ha; 240 lb/acre) actually increased spring dead spot incidence. Application of excessive amounts of potassium or other nutrients, beyond what is required for optimal turf growth, is not recommended.
Other turfgrass diseases caused by ectotrophic root-infecting fungi, such as take-all patch (Gaeumannomyces graminis var. avenae) and summer patch (Magnaporthe poae), are strongly influenced by soil pH (19). These diseases are suppressed by reductions in soil pH following application of elemental sulfur or acidifying nitrogen sources such as ammonium sulfate (7). It has long been speculated that spring dead spot is similarly influenced by soil pH, but there is little scientific support for this relationship. Severe cases of spring dead spot are commonly observed in soils with pH ranging from less than 5 to over 8. In Maryland, monthly applications of ammonium sulfate (NH4SO4) + potassium chloride (KCl) from May through September at 1 lb of N per 1000 ft² (49 kg/ha) and 0.8 lb of K per 1000 ft² (39 kg/ha) reduced spring dead spot the following spring by up to 41%, possibly due to acidification of the rhizosphere from ammonium sulfate (8). On the other hand, recent research in North Carolina indicates that spring dead spot severity is greatest in plots irrigated with pH 3 irrigation water, and lowest when irrigated with pH 7 irrigation water (Fig. 19).
| |

Fig. 19. Spring dead spot average patch diameter in response to irrigation water pH. During summer 2006, plots were irrigated weekly with 0.25 inch of water, which was adjusted to the appropriate pH using sulfuric acid or sodium hydroxide. Data was collected in May 2007. |
|
Field evaluations of fungicides for the control of spring dead spot have dated back to the 1960s (7). Products such as nabam, carboxin, chloroneb, maneb, benomyl, chlorothalonil, PCNB, thiram, tebuconazole, diniconazole, fenarimol, propiconazole, and thiophanate-methyl have all been tested for efficacy against spring dead spot with variable results among researchers and geographic regions. Curently, fenarimol, myclobutanil, azoxystrobin, thiophanate-methyl, and propiconazole are labeled for spring dead spot control, with fenarimol and myclobutanil being the most commonly applied.
In North Carolina, Wetzel (50) tested fungicides for preventative control of spring dead spot in ‘Tifway’ bermudagrass fairways. Only fenarimol provided significant control of spring dead spot in this study, reducing spring dead spot severity by 71 to 85% and increasing turfgrass quality. Also in North Carolina, Tredway and Butler (38) reported a significant reduction in spring dead spot severity from two fall applications of Patchwork 0.78G (4 lb/1000 ft²; 195 kg/ha) and Rubigan 1AS (4 fl oz/1000 ft²; 13 liter/ha), which both contain the active ingredient fenarimol. An experimental DMI fungicide, tebuconazole, has also shown good to excellent efficacy against spring dead spot in North Carolina (40). In Oklahoma, Walker et al. (46) reported no significant control of spring dead spot from preventative application of all fungicides examined. In another study by Walker (44), propiconazole, tebuconazole, and fenarimol all significantly reduced spring dead spot severity; however, the frequency of fungicide application was cost prohibitive.
Few specific recommendations for preventative control of spring dead spot are available to turfgrass managers. In fact, several state extension publications do not recommend preventative fungicide applications for spring dead spot control due to erratic results (24,35,41). Effective and consistent control of spring dead spot will not be possible until we develop comprehensive understanding of the conditions that may influence fungicide performance, including product selection, application timing, application techniques, soil conditions, and the pathogens species present.
Butler and Tredway completed a comprehensive investigation of spring dead spot control in North Carolina (4). In a series of experiments over three years, fenarimol provided the most effective and consistent control of spring dead spot caused by O. korrae. Propiconazole provided significant control in some trials, whereas myclobutanil and azoxystrobin were ineffective. They also employed a variety of application methods, including standard foliar sprays, watered-in applications, or high pressure soil injection, and found no significant effect on fungicide performance. Fenarimol (Rubigan 1AS) was equally effective when applied in the fall across a broad range of soil temperatures, between 60°F and 80°F (15.5°C and 26.7°C), and single applications at 6 fl oz/1000 ft² (19 liter/ha) were as effective as two applications at either 4 fl oz or 6 fl oz/1000 ft² (13 or 19 liter/ha).
In general, attempts to control spring dead spot in the Midwestern U.S. with fungicides have been less successful than those in the Eastern U.S. Some have speculated that this is due to the increased virulence of O. herpotricha, which is dominant in the Midwest, as compared to O. korrae, the dominant species in the Eastern U.S. However, recent research in North Carolina demonstrates that both species are controlled effectively by fenarimol, tebuconazole, and other fungicides (Fig. 20). Additional factors, such as climatic conditions or soil properties, may be responsible for differences in fungicide performance across geographic regions.
| |
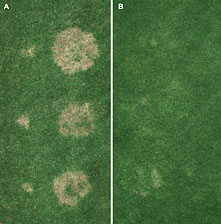
Fig. 20. Control of spring dead spot caused by O. korrae andO. herpotricha in North Carolina: (A) untreated; (B) two fall applications of fenarimol (Rubigan) at 4 fl oz/1000 ft². Each plot was inoculated with a three-isolate mixture of O. korrae (three small patches, left side of plot) and O. herpotricha (three large patches, right side of plot). |
|
Outlook for the Future
Despite the many desirable traits of bermudagrasses, spring dead spot continues to limit the quality of turfgrass areas established with these species. Through the efforts of many researchers over the last 60 years, we have begun to understand the individual factors that influence spring dead spot development, including pathogen distribution and virulence, cultivar susceptibility, winter dormancy, freezing temperatures, soil moisture, fertilization practices, and fungicide applications. However, spring dead spot occurrence is driven by interactions among all of these factors, so an understanding of individual factors in isolation is insufficient. Future research must focus on these interactions in order to develop disease management programs that are specific, effective, and economical.
Literature Cited
1. Baird, J. H., Martin, D. L., Taliaferro, C. M., Payton, M. E., and Tisserat, N. A. 1998. Bermudagrass resistance to spring dead spot caused by Ophiosphaerella herpotricha. Plant Dis. 82:771-774.
2. Burton, G. W., and Powell, J. B. 1971. Better bermudagrasses to meet golf's demands. USGA Green Sec. Rec. 9:9-11.
3. Butler, E. L. 2004. Development of novel strategies for control of spring dead spot in hybrid bermudagrass. M.S. thesis. North Carolina State Univ., Raleigh, N.C.
4. Butler, E. L., and Tredway, L. P. 2006. Method and timing of fungicide applications for control of spring dead spot in hybrid bermudagrass. Online. Plant Health Progress doi:10.1094/PHP-2006-0901-01-RS.
5. Caasi, O. C., Walker, N. R., Marek, S. M., and Mitchell, T. K. 2007. Infection and colonization of three turf-type bermudagrass cultivars by Ophiosphaerella herpotricha expressing green fluorescent protein. Phytopathology S97:16.
6. Crahay, J. N., and Dernoeden, P. H. 1988. Growth and pathogenicity of Leptosphaeria korrae in bermudagrass. Plant Dis. 72:945-949.
7. Dernoeden, P. H. 1993. Integrating strategies for the management of patch diseases caused by root invading ectotrophic fungi. Pages 123-161 in: Turfgrass Patch Diseases Caused by Ectotrophic Root-Infecting Fungi. B. B. Clarke and A. B. Gould, eds. American Phytopathological Society, St. Paul, MN.
8. Dernoeden, P. H., Crahay, J. N., and Davis, D. B. 1991. Spring dead spot and bermudagrass quality as influenced by nitrogen source and potassium. Crop Sci. 31:1674-1680.
9. Elliott, M. L. 1991. Determination of an etiological agent of bermudagrass decline. Phytopathology 81:1380-1384.
10. Endo, R. M., Ohr, H. D., and Krausman, E. M. 1985. Leptosphaeria korrae, a cause of the spring dead spot disease of bermudagrass in California. Plant Dis. 69:235-237.
11. Green, D. E. I., Fry, J. D., Pair, J. C., and Tisserat, N. A. 1993. Pathogenicity of Rhizoctonia solani AG-2-2 and Ophiosphaerella herpotricha on zoysiagrass. Plant Dis. 77:1040-1044.
12. Hammer, W., and Chastagner, G. 1987. Factors affecting the production of pseudothecia of Leptosphaeria korrae in vitro. Phytopathology 77:1239.
13. Henry, A. W. 1932. Influence of soil temperature and soil sterilization on the reaction of wheat seedlings to Ophiobolus graminis. Can. J. Res. 7:198-203.
14. Iriarte, F. B., Todd, T. C., Tisserat, N. A., Fry, J. D., and Martin, D. L. 2005. Effect of cold acclimation and freezing on spring dead spot severity in bermudagrass. HortScience 40:421-423.
15. Iriarte, F. B., Wetzel, H. C., Fry, J. D., Martin, D. L., Vincelli, P., Dixon, E. W., and TIsserat, N. A. 2005. Aggressiveness of spring dead spot pathogens to bermudagrass. Intnl. Turfg. S. Res. J. 10:258-264.
16. Iriarte, F. B., Wetzel, H. C. I., Fry, J. D., Martin, D. L., and Tisserat, N. A. 2004. Genetic diversity and aggressiveness of Ophiosphaerella korrae, a cause of spring dead spot of bermudagrass. Plant Dis. 88:1341-1346.
17. Jackson, N. 1993. Geographic distribution, host range, and symptomology of patch diseases caused by soilborne ectotrophic fungi. Pages 17-39 in: Turfgrass Patch Diseases Caused by Ectotrophic Root-Infecting Fungi. B. B. Clarke and A. B. Gould, eds. American Phytopathological Society, St. Paul, MN.
18. Kozelnicky, G. M. 1974. Updating 20 years of research: Spring dead spot. USGA Green Sec. Rec. (May):12-15.
19. Landschoot, P., Gould, A. B., and Clarke, B. B. 1993. Ecology and epidemiology of ectotrophic root-infecting fungi associated with patch diseases of turfgrasses. Pages 73-105 in: Turfgrass Patch Diseases Caused by Ectotrophic Root-Infecting Fungi. B. B. Clarke and A. B. Gould, eds. American Phytopathological Society, St. Paul, MN.
20. Lucas, L. T. 1980. Spring dead spot of bermudagrass. USGA Green Sec. Rec. 18:4-6.
21. Lucas, L. T., and Gilbert, W. B. 1979. Managing dormant bermudagrass. Golf Course Manag. 47:14-16, 21-23.
22. Madison, J. H. 1970. Do we have a new turf grass disease? J. Sports Turf Res. Inst. 46:17-21.
23. Maples, P. J. 2001. Soaring with bermudagrass: A history of Southern grasses. Golf Course Manag. 69:33-36.
24. Martin, D., and Hudgins, B. Managing spring dead spot disease of bermudagrass. Online. Coop. Ext. Serv. Publ. No. EPP-7665, Oklahoma State Univ., Stillwater, OK.
25. Martin, D. L., Bell, G. E., Baird, J. H., Taliaferro, C. M., Tisserat, N. A., Kuzmic, R. M., Dobson, D. D., and Anderson, J. A. 2001. Spring dead spot resistance and quality of seeded bermudagrasses under different mowing heights. Crop Sci. 41:451-456.
26. McCarty, L. B., DiPaola, J. M., and Lucas, L. T. 1991. Regrowth of bermudagrass infected with spring dead spot following low temperature exposure. Crop Sci. 31:182-184.
27. McCarty, L. B., and Lucas, L. T. 1989. Gaeumannomyces graminis associated with spring dead spot of bermudagrass in the southeastern United States. Plant Dis. 73:659-661.
28. McCarty, L. B., Lucas, L. T., and DiPaola, J. M. 1992. Spring dead spot occurrence in bermudagrass following fungicide and nutrient applications. HortScience 27:1092-1093.
29. McCarty, L. B., and Miller, G. L. 2002. Managing Bermudagrass Turf. Ann Arbor Press, Chelsea, MI.
30. Nus, J. L., and Shashikumar, K. 1993. Fungi associated with spring dead spot reduce freezing resistance in bermudagrass. HortScience 28:306-307.
31. Sauer, K. M., Hulbert, S. H., and Tisserat, N. 1993. Identification of Ophiosphaerella herpotricha by cloned DNA probes. Phytopathology 83:97-102.
32. Shoemaker, R. A., and Babcock, C. E. 1989. Phaeosphaeria. Can. J. Bot. 67:1500-1599.
33. Smith, A. M. 1971. Control of spring dead spot of couch grass turf in New South Wales. J. Sports Turf Res. Inst. 47:60-65.
34. Tisserat, N., III, H. W., Fry, J., and Martin, D. L. 1999. Spring dead spot of Buffalograss caused by Ophiosphaerella herpotricha in Kansas and Oklahoma. Plant Dis. 83:199.
35. Tisserat, N. A. Spring dead spot of bermudagrass. Online. Agric. Expn. Stn. and Coop. Ext. Serv. Kansas State University, Manhattan, KS.
36. Tisserat, N. A., Hulbert, S. H., and Sauer, K. M. 1994. Selective amplification of rDNA internal transcribed spacer regions to detect Ophiospaerella korrae and O. herpotricha. Phytopathology 84:478-482.
37. Tisserat, N. A., Pair, J. C., and Nus, A. 1989. Ophiosphaerella herpotricha, a cause of spring dead spot of bermudagrass in Kansas. Plant Dis. 73:933-937.
38. Tredway, L. P., and Butler, E. L. 2004. Evaluation of fungicides for control of spring dead spot, 2003-2004. Fung. Nemat. Tests 60:T014.
39. Tredway, L. P., and Butler, E. L. 2007. First report of spring dead spot of zoysiagrass caused by Ophiosphaerella korrae in the United States. Plant Dis. 91:1684.
40. Tredway, L. P., Butler, E. L., Soika, M. D., and Bunting, M. L. Etiology and management of spring dead spot of hybrid bermudagrass in North Carolina, USA. Acta Horticulturae (In press).
41. Vincelli, P. Managing spring dead spot of bermudagrass. Coll. of Agric. Publ. No. ID-130, University of Kentucky, Lexington, KY.
42. Wadsworth, D. F., and Young, H. C. 1960. Spring dead spot of bermudagrass. Plant Dis. 44:516-518.
43. Walker, J., and Smith, A. M. 1972. Leptosphaeria namari and L. korrae sp. nov., two long-spored pathogens of grasses in Australia. Trans. Brit. Mycol. Soc. 58:459-466.
44. Walker, N. R. 2004. Evaluation of fungicides for the management of spring dead spot of bermudagrass, 2003-2004. Fung. Nemat. Tests 60:T022.
45. Walker, N. R.. 2006. Viability of dormant bermudagrass in spring dead spot patches caused by Ophiosphaerella herpotricha during winter months. Phytopathology S96:119.
46. Walker, N. R., Jackson, K. E., and Martin, D. L. 2001. Evaluation of fungicides for the management of spring dead spot of common bermudagrass turf, 2000-2001. Fung. Nemat. Tests 57:T12.
47. Walker, N. R., Mitchell, T. K., Morton, A. N., and Marek, S. M. 2006. Influence of temperature and time of year on colonization of bermudagrass roots by Ophiosphaerella herpotricha. Plant Dis. 90:1326-1330.
48. Webster, J., and Hudson, H. J. 1957. Graminicolous pyrenomycetes. VI. Conidia of Ophiobolus herpotrichus, Leptosphaeria luctosa, L. fuckelii, L. pontiformis and L. eustomoides. Trans. Brit. Mycol. Soc. 40:459-466.
49. Wetzel, H. C. 1999. Geographic distribution and genetic diversity of three Ophiosphaerella species that cause spring dead spot of bermudagrass. Plant Dis. 83:1160-1166.
50. Wetzel, H. C. 2000. Evaluation of fungicides for control of spring dead spot of bermudagrass, 2000. Fung. Nemat. Tests 56:T3.
51. Wetzel, H. C., III, Hulbert, S. H., and Tisserat, N. A. 1999. Molecular evidence for the presence of Ophiosphaerella narmari n. comb., a cause of spring dead spot of bermuda grass, in North America. Mycol. Res. 103:981-989.
52. Young, H. C., Sturgeon, R. V. J., and Huffine, W. W. 1973. Soil type associated with spring dead spot of bermudagrass. Phytopathology 63:450.
