"A highly contagious fungus that destroys tomato plants has quickly spread to
nearly every state in the Northeast and the mid-Atlantic, and the weather over
the next week may determine whether the outbreak abates or whether tomato
crops are ruined…"
The New York Times, 18 July 2009, J. Moskin
"You say tomato, I say agricultural disaster."
The New York Times, 9 August 2009, D. Barber
Indeed, the tomato late blight pandemic of 2009 made late blight into a household term in much of eastern USA. Many home gardeners and many organic producers lost most, if not all, of their tomato crop. Some CSAs (Community Supported Agriculture) could not provide tomatoes to their members. In response, many questions emerged: How did it happen? What was unusual about this event compared to previous late blight epidemics? What is the current situation in 2012, and what can be done?

Fig. 1. Infected tomato transplants. This image was taken of transplants for sale in a retail store in June in 2009. |
|
This pandemic was unusual. It started synchronously in mid to late June over much of the northeastern USA. The pathway was via infected tomato transplants (Figs. 1, 2, and 3) shipped to garden centers in large retail stores throughout the Northeast. The fact that infected transplants were being sold in such stores from Pennsylvania to Maine became abundantly clear by late June 2009. Most employees in the garden centers did not recognize the symptoms of late blight, and most home gardeners also were unaware of the disease and planted infected transplants into their gardens. The first warning to the plant pathology community in the Northeast was from Andy Wyenandt who, on 18 June, reported late blight on tomato transplants for sale in a garden center in New Jersey. This report was shared widely with plant pathologists in the Northeast via a "Late blight" email listserv organized by Abby Seaman in the New York State IPM program. This report was followed shortly by a report from Meg McGrath who reported late blight on commercial potatoes on Long Island (23 June). However, the seriousness of the situation was not fully appreciated until 24 to 26 June, when plant pathologists in New York and Maine found infected tomato transplants in many big box stores. The note from Tom Zitter on 26 June (Fig. 4) described the situation in Ithaca, NY. Zitter and others reported that transplants in big retail ("box") stores supplied by the same single national supplier were infected, but transplants in local garden centers which had obtained tomato transplants from local sources were free of late blight. From 24 to 30 June, we know of 49 reports to the listserv and the Northeast Plant Disease Network – 37 described infected transplants being sold in big box stores. Extension staff in the Northeast were alerted, and in New York almost all counties had reported late blight by mid August (Fig. 5). In many cases, extension personnel informed store managers that the infected transplants were dangerous to home gardens (Figs. 6 and 7), organic farms, and conventional crops. In some cases, store managers were helpfully responsive. However, because the transplants were on consignment (the store did not own them), some managers felt that they could not summarily destroy the plants and had to depend on the supplier to remove the transplants. From 23 June to 29 July, we know of 155 reports of late blight from throughout the Northeast (Fig. 8). Reports of late blight on infected transplants in stores ceased after mid July.
 |
|
 |
|
Fig. 2. Foliar late blight lesions on tomato transplants for sale in a garden center in 2009. |
|
Fig. 3. Fruit infections on a large tomato patio tomato for sale in a garden center in 2009. |
| |

Fig. 4. Message from Tom Zitter to the extension community on 26 June 2009. |
|
| |
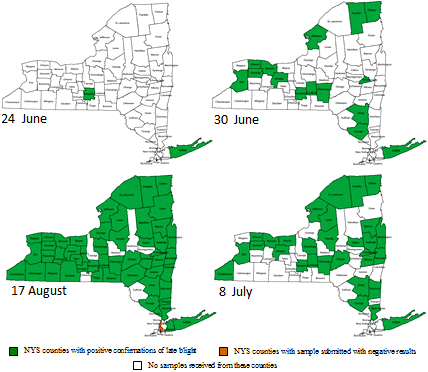
Fig. 5. Counties in New York which had reported late blight (in a garden center or in the field) during the summer 2009. (Thanks to the NPDN for constructing these maps.) |
|

A |
|

B |
|
Fig. 6. Late blight on tomatoes in a community garden in Ithaca, NY, on 9 July 2009. |
| |

Fig. 7. Late blight on potatoes in the same garden plot as the tomatoes in Figure 6. |
|
| |
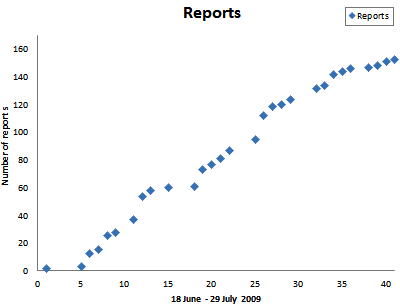
Fig. 8. Reports of late blight to the Cornell Diagnostic Lab from 18 to 29 July 2009. |
|
Weather in the Northeast was relatively favorable to late blight development during June and July 2009. For example, in central New York there was a rapid accumulation of "severity values" on the Blitecast forecast system from late June to early-mid July (Fig. 9). (Severity values are a measure of how favorable the weather has been for late blight development. For example, if fewer than four Blitecast "severity values" occurred in the past week, there is a "no fungicide spray" recommendation, but if more than seven severity values were recorded, a fungicide schedule of every five days is recommended.) This weather enabled P. infestans in infected transplants planted into home gardens to sporulate, disperse to, and infect neighboring potatoes and tomatoes (Fig. 10). A pandemic was initiated, and it caught the attention of the general population in the Northeast (1,7). Most early reports of late blight in the field were from home gardens and organic farms. Commercial potatoes were largely unaffected in early 2009 – probably because commercial potato growers typically apply fungicides.
| |
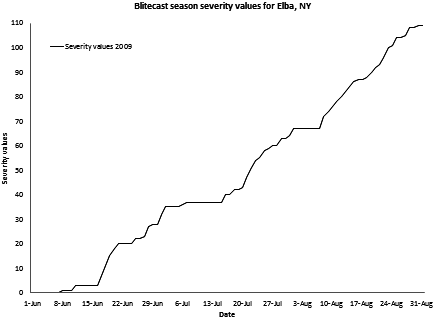
Fig. 9. Cumulative Blitecast severity values in upstate New York (Elba) in summer 2009. Severity values are a measure of how favorable the weather has been for late blight development. For example, if fewer than four Blitecast "severity values" occurred in the past week, there is a "no fungicide spray" recommendation, but if more than seven severity values were recorded, a fungicide schedule of every five days is recommended. |
|
| |

Fig. 10. Severely late blighted tomatoes on an organic farm near Ithaca, NY, in late July 2009. These plants were the last tomatoes on the farm. All others had been destroyed by late blight. These plants were scheduled to be removed within hours of when the image was taken. |
|
Prior to 2009 (and in most subsequent outbreaks) late blight occurred in production fields where fungicide applications were applied unevenly and rows or edges were missed. Extension personnel were surprised in 2009 because this outbreak was entirely different. Suddenly the disease was widespread on tomatoes in home gardens and in retail outlets throughout the Northeast. Communicating with the home gardener audience was a challenge because this audience is diverse and widespread, and often served by personnel who do not also serve commercial growers. Extension personnel quickly strengthened linkages with extension home and garden agents to educate the public about the need to take prompt action to protect crops.
Many homeowners and organic growers lost crops when fungicide was not applied soon enough, and because they lacked highly effective curative fungicide options. (Conventional commercial growers, who have more fungicide options, were more successful in delaying the epidemic and subsequent yield loss.) Other growers mistakenly thought their crops in high tunnels were protected. Persons who understood the potential of this disease panicked because they did not know how close they were to a source of inoculum. Many organic growers and home gardeners lacked experience with late blight, and were unfamiliar with the appropriate fungicides. In some locations rain occurred relentlessly during the start of the pandemic and even knowledgeable persons became concerned because they could not get out to apply fungicide, or because rains removed fungicide immediately after application. Because tomatoes are extremely important to the financial success of many smallholder vegetable growers, some of these persons panicked as they observed the rapid destruction of their crops. There was widespread anger that the situation occurred and that sales of infected plants were not been halted more quickly.
Dismay was felt by many plant pathologists and extension personnel who tried unsuccessfully to stop sales of infected transplants in garden centers by explaining the seriousness of late blight to managers of stores selling symptomatic tomatoes. However, most persons in these stores had no comprehension of a plant disease and could not understand the implications of selling a diseased tomato plant from the garden center of a big box store. When admonished to remove infected transplants, one store manager responded by putting them on sale! Extension professionals expended tremendous effort and time into educating gardeners about late blight. Numerous web postings and flyers were produced. Press releases captured the attention of the media and public and extension professionals provided many interviews for the written and broadcast media.

Fig. 11. Email message from a very disappointed tomato grower, whose tomato crop had been completely devastated by late blight by late July 2009. |
|
Denial, surprise, panic, confusion, anger, and concern were among responses from the public. Tomatoes are a crop regarded passionately by many gardeners. Once they understood the implications of late blight, they were horrified to learn that they had planted infected transplants in their gardens. Unfortunately, many gardeners learned about the devastation of late blight by observing their tomato plants dying – seemingly overnight (Fig. 11). There was mass confusion, initially because most gardeners (even very experienced ones) had never seen late blight. Some wondered if there might be health issues from breathing pathogen spores while tearing out diseased plants. There was some anger over the news that diseased plants were sold. Stores in one town are known to have offered refunds. Later in the year other issues arose. Consumers were alarmed to see fungicide residues on tomatoes at farmers markets, especially on organically produced fruit. (Some copper-based fungicides are allowed in organic production in some states.) Consumers sought answers about the nature of the residues and the implications to their health. Gardeners who had healthy plants until harvest had many questions: Were there any health concerns about consuming symptomless portions of affected fruit? Could the fruit be canned? How should they dispose of diseased tissue? How should they treat the soil? Upon destroying infected plants, some gardeners abandoned the remainder of the garden; others burned the clothing they wore when destroying the infected plants. Some annual tomato festivals were canceled.
Some fungicides, notably those approved for organic production, were sometimes not readily available. Agricultural chemical manufacturers and distributors had not previously experienced such a pandemic and were as surprised as everyone else. However, federal and state agencies responsible for pesticide registrations promptly responded to the low supply of some fungicides and they approved at least one supplemental label. Some organic growers were unable to effectively manage late blight partly because of pesticide use restrictions.
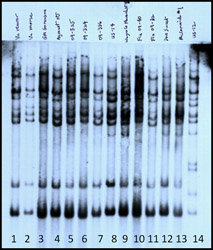
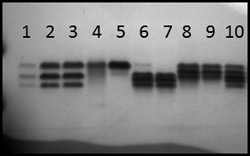
Because infected transplants in June 2009 were associated with a single supplier throughout the Northeast, it seemed important to determine if the isolates causing late blight in these box stores were similar to each other or different from each other. Many samples of infected tissues were shipped to Cornell during the summer. These were assayed for allozymes at the glucose-6-phosphate isomerase (Gpi) locus using sporangia from sporulating lesions. Using sporangia from a sporulating lesion, this assay can be completed in just a few hours (4). All samples from big box stores, from organic farms, and from home gardens had the same (Gpi) genotype (100/122). A more informative technique was DNA fingerprinting using fingerprint probe RG57 (3). However, this technique requires a significant amount of pathogen tissue (for DNA extraction), so we isolated the pathogen into pure culture from many samples. We then also assayed these cultures for mating type and mefenoxam sensitivity. As the results gradually came in over the summer, we learned that all of the isolates from box stores, home gardens, and organic farms were indistinguishable from each other: they were all A2 mating type, had the same DNA fingerprint (Fig. 12), had the same Gpi genotype (Fig. 13), and were sensitive to mefenoxam. Unfortunately, these additional data did not become available until the epidemic was well established. The pandemic strain was termed US22 (5) (Table 1).
 |
|
 |
|
Fig. 12. Southern blot of DNA from several isolates of P. infestans detected by RG57. Each column contains the DNA of a single isolate. The DNA had been cut with restriction enzyme EcoR1 and then fragments were separated on an agarose gel. This analysis was conducted in July 2009. Lanes 8 and 14 are controls; Lane 9, 12, and 13 are US8; and Lanes 1-7 and 10-11 are US22. |
|
Fig. 13. Polymorphism at the Glucose-6-phosphate isomerase locus. Each lane contains proteins from a single isolate of P. infestans. The proteins are separated via migration in an electric field. The bands are developed via an enzyme assay. The genotypes of the individuals in the diverse lanes are as follows: Lanes 1-3 are 100/122; Lanes 4 & 5 are 100/100; Lanes 6 & 7 are 111/122; Lanes 8 & 9 are 100/111; and Lane 10 is 100/111/122. |
Table 1 . Characteristics of clonal lineages recently detected in the USA.
| Lineage |
Mating type |
mtDNA |
Host
specialization |
Glucose-6
phosphate
isomerase |
Mefenoxam
sensitivity |
| US8 |
A2 |
Ia |
Potato |
100/111/122 |
I-R |
| US11 |
A1 |
Ia |
Potato/Tomato |
100/111 |
R |
| US22 |
A2 |
Ia |
Potato/Tomato |
100/122 |
S |
| US23 |
A1 |
Ia |
Potato/Tomato |
100/100 |
S |
| US24 |
A1 |
Ia |
Potato |
100/100/111 |
S (some I)* |
*Most individuals of US24 appear sensitive (S) to mefenoxam, but others appear intermediate (I) in their sensitivity. US11 has been consistently resistant (R). Data are from (2).
When we learned in late July 2009 that the strain causing the pandemic was sensitive to mefenoxam, it became clear that a technique to rapidly identify this particular strain was highly desired. The allozyme assay was insufficient because, although it could be applied to sporangia from a sporulating lesion on the day of receipt in a lab, and results could be obtained in a few hours, the assay was insufficiently discriminatory. Other diverse strains could have the same allozyme genotype by chance. We therefore initiated an analysis of microsatellite markers to assess diversity among the strains, employing the procedure described by Lees et al. that interrogates genotypes at more than 10 loci (6). Microsatellite markers are PCR-based and therefore can be employed on small samples (sporangia from lesions) and therefore if one starts with sporangia from a lesion, the results can often be obtained in 24 h. Using the Lees et al. protocol we were able to obtain a multilocus genotype that separated the 2009 pandemic strain from other recent strains (Fig. 14). Thus, microsatellites became a very useful tool to rapidly identify specific clonal lineages. If the phenotypic characteristics of a strain were already known, one could use microsatellites to rapidly predict the traits of the pathogen in a sample.
| |
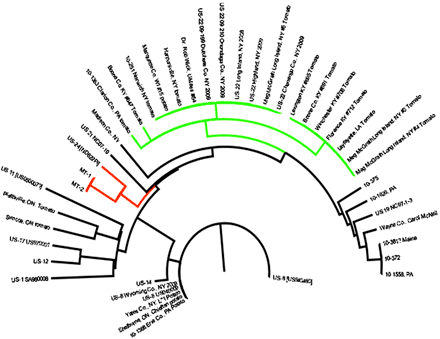
Fig. 14. Dendrogram illustrating the relationships among the recent clonal lineages of P. infestans collected in the USA as revealed using microsatellite markers. Isolates associated with the green branches are US22. Isolates with red branches are US24. Data are from Small et al. (unpublished).
|
|
While US22 dominated the population in the Northeast in 2009, a few other clonal lineages were also detected. US8 (Table 1), was detected in a few samples from commercial potatoes. After the summer of 2009 and upon assessment of additional samples, it became clear that another lineage (US23) was present in the east (Pennsylvania) and south. However, US23 did not appear to have been distributed on tomato transplants, and was not detected in northeastern USA during the summer 2009. Finally, another new strain (US24) was reported on potatoes from the upper Midwest. US8, US22, US23, and US24 can be distinguished from each other by their allozyme genotype (Table 1), RG57 fingerprint, and microsatellite genotype. The phenotypes of these lineages are also distinct from each other (see below).
Isolates of four lineages (US22, US23, US24, and US8) collected in 2010 were investigated for phenotypic characteristics such as pathogenicity on potato and tomato (Fig. 15), sensitivity to mefenoxam, and speed of germination (2) . These isolates (total n = 59) came from many different locations (in 12 states and one Canadian province). The number of isolates of each lineage ranged from 8 to 35. These laboratory assessments confirmed preliminary field observations. First, both US22 and US23 are pathogenic to both potato and tomato. However, it seems that US23 might be even more aggressive than US22 on both potatoes and tomatoes. This discovery supports the conclusion that a major reason for the pandemic of 2009 was that US22 was so widely and efficiently dispersed on tomato transplants – not that US22 is more aggressive than other strains. US22 and US23 are also fairly sensitive to mefenoxam. US24 is pathogenic mainly on potatoes, and not at all aggressive to tomato. These pathogenicity characteristics are similar to those of US8. Most individuals of US24 are sensitive to mefenoxam, but there may be some diversity in this lineage for mefenoxam sensitivity [see (2) for details]. US8 has been previously characterized and the 2010 isolates (Table 1) were shown to have characteristics similar to isolates collected in the 1990s. The phenotypic characterization has only just begun, and there may be other important differences among the lineages. For example we have recently learned that US22 and US23 release zoospores more slowly than do US8 and US24 (2) .
| |
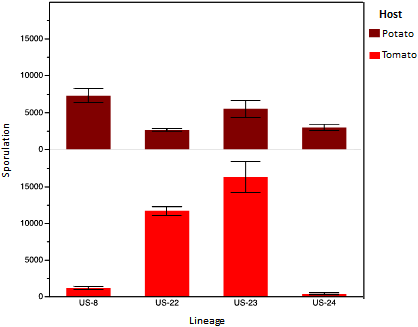
Fig. 15. Pathogenicity of different recent lineages of P. infestans on potato and tomato. The numbers of isolates involved in the assessments were: US22 – 35; US23 – 7; US24 – 9; US8 – 8. Data are from Danies et al. (2). The graph shows the mean ± standard error of sporulation counts. |
|
We were very interested in determining whether or not the expression of a particular trait within a lineage was consistent among isolates of that lineage. If it is consistent, then we will be able to make predictions of phenotype based on knowledge of genotype (lineage). For the 59 isolates in the four lineages investigated by Danies et al. (2), it appears that isolates within most lineage have fairly consistent phenotypes. However, for the in vitro mefenoxam assay, some isolates in lineage US24 appeared to be sensitive to mefenoxam whereas others were resistant.
The epidemic of 2009 reawakened interest in late blight. In 2010, this interest coincided with an opportunity from USDA National Institute of Food and Agriculture to submit a research-extension proposal focused on oomycetes. Such a proposal (with >25 co-PIs, including participants from 13 states in the USA and two other countries) was constructed and submitted to the USDA. Fortunately, the proposal was funded and the project started in March 2011. This grant supports many efforts in extension and research (ranging from very basic to very applied). Because US22 appears to be thwarted by tomato resistance genes Ph2 and Ph3, tomato cultivars with these resistances have been targeted for rapid development. Other approaches to developing host resistance are also being investigated. Some of the other very basic research has the practical goal of developing rapid diagnostic tools – such as a rapid diagnostic test for mefenoxam resistance. Participating extension plant pathologists are not only heavily involved in education but also in rapid diagnosis and reporting. These plant pathologists have agreed to report occurrences of late blight to the USAblight website (http://usablight.org) and to send samples for genotypic analyses. Because of the explosive potential of late blight, early warning is crucial to effective suppression.
This review began by asking several questions. We return here to answer those questions.
(i) "How did the 2009 pandemic happen?" The pandemic was initiated by a synchronous region-wide release of one strain of the pathogen into the environment on infected tomato transplants bought by home gardeners who did not know about late blight. The subsequent weather was favorable to the pathogen and susceptible hosts were readily available – so that all requirements necessary for a pandemic were met.
(ii) "What was unusual about this epidemic?" In our experience, the scale of pathogen release was completely unexpected and unprecedented. The pathogen was released via infected tomato transplants throughout the entire Northeast at the same time. No one was prepared for this avenue of pathogen distribution on such a large scale. Obviously, if one aspect of the system is dominantly influenced by a single supplier, then any mistake may have significant consequences.
(iii) "What is the current situation and what can be done?" We have described the current situation above, and we believe that new understanding, education, and communication have contributed greatly to the effective management of this disease in 2010 and 2011 compared to 2009. Future research needs to be multifaceted with the understanding that integrated, multifaceted strategies have the best chance for success.
Many new technologies and approaches are needed to realize more effective late blight suppression in the future. These include: improved communications, better weather forecasts, improved knowledge of aerial dispersal of P. infestans, improved diagnostics, new resistant potato cultivars, new resistant tomato cultivars, and new approaches to achieving host resistance. Efforts to meet some of these needs have been initiated. Of all of these needs, it is particularly high priority to improve diagnostics for specific traits of the pathogen. This is because, although use of microsatellite and allozyme markers to identify a particular lineage is currently effective to predict important phenotypic traits in the highly clonal population in the USA, that approach is unlikely to always be effective. For example, this approach will not be effective for a population into which new genotypes (with new phenotypes) are introduced. The most likely pathways for the introduction of new genotypes are via migration and via sexual recombination. Some migration is expected. However, perhaps the more important pathway will be via sexual recombination in the P. infestans population in the USA. It seems highly likely that eventually the population of P. infestans in the USA will become sexual. Thus, one goal of the AFRI project is to develop rapid specific diagnostics for the actual trait. For example, if the gene responsible for mefenoxam resistance were known, one could develop a PCR-based assay that would be a direct diagnosis. Additionally, if the effectors recognized by Ph2 and Ph3 were known, one could assay for these effectors using molecular tools.
Fortunately, there is research all over the world on potato and tomato late blight, and technologies and approaches developed in one location can be rapidly adopted elsewhere. Exciting new developments in our understanding of the genome of the pathogen, of plant responses, and of the biology, ecology and epidemiology of the pathogen have converged to stimulate integrated projects on late blight all over the world. It is important to emphasize to growers and policy makers that an integrated approach is needed and to remind them that previous reliance on single "silver bullet" approaches have always been transitory.
1. Barber, D. 2009. You say tomato, I say agricultural disaster. WK10, 9 August 2009, New York Times.
2. Danies, G., Small, I. M., Myers, K., Zuluaga, A. P., Childers, R., Becoscke, K., Stead, S., Teerantanonon, A., D'Attilio, D., and Fry, W. E. 2012. Phenotypic and genotypic characterization of recent clonal lineages of Phytophthora infestans in the United States and Canada. Phytopathology (In press).
3. Goodwin, S. B., Drenth, A., and Fry, W. E. 1992. Cloning and genetic analyses of two highly polymorphic, moderately repetitive nuclear DNAs from Phytophthora infestans. Curr. Genet. 22:107-115.
4. Goodwin, S. B., Schneider, R. E., and Fry, W. E. 1995. Use of cellulose-acetate electrophoresis for rapid identification of allozyme genotypes of Phytophthora infestans. Plant Dis. 79:1181-1185.
5. Hu, C.-H., Perez, F., Donohoo, R., McLeod, A., Myers, K., Ivors, K., Secor, G., Roberts, P., Deahl, K., Fry, W. E., and Ristaino, J. B. 2012. Recent genotypes of Phytophthora infestans in eastern USA reveal clonal populations and reappearance of mefenoxam sensitivity. Plant Dis. 96:1323-1330.
6. Lees, A. K., Wattier, R., Shaw, D. S., Sullivan, L., Williams, N. A., and Cooke, D. E. 2006. Novel microsatellite markers for the analysis of Phytophthora infestans populations. Plant Pathol. 55:311-319.
7. Moskin, J. 2009. Outbreak of fungus threatens tomato crop. A16, 18 July 2009, New York Times.
