Introduction
In 1992, Hawaii’s papaya industry faced a potential economic disaster when Papaya ringspot virus (PRSV) was discovered in the Puna district of Hawaii island where 95% of the state’s papaya was grown (3). By 1995, PRSV was widespread in Puna and the industry was in a crisis situation (Figs. 1A and 1B). Fortunately, our research had resulted in the development of a transgenic papaya that was resistant to PRSV; in fact, an initial field trial of the transgenic papaya was established on Oahu island at about the time PRSV was discovered in Puna (2,6). An APSnet Feature published in 1998 described the ensuing damage caused by PRSV in Puna and the timely efforts to develop, evaluate, and deregulate the transgenic papaya (5). SunUp and Rainbow cultivars were developed from the initial field trial and showed excellent resistance and horticultural qualities in the large scale field trial in Puna. The cultivars were commercially released in May 1998, six years to the month after PRSV was discovered in Puna. The 1998 APSnet Feature was appropriately titled "Transgenic Virus Resistant Papaya: New Hope for Control of Papaya Ringspot Virus in Hawaii." The industry was full of hope then, but it remained to be seen whether the transgenic papaya would translate this hope to a reality. Six years have transpired since the commercialization of the transgenic papaya. In this article, we describe the successful performance of the transgenic papaya in Hawaii, its impact, and challenges facing Hawaii’s papaya industry. A subsequent APSnet Feature will provide details on research done to document the adoption of the transgenic papaya by farmers soon after it was released.

A |
|

B |
Fig. 1. (A) Healthy Puna papaya fields in 1992;
(B) Severely PRSV-infected papaya fields in 1994 that were abandoned.
PRSV in Hawaii and Transgenic Papaya to 1998
Although present in Hawaii since the 1940s, PRSV was a nuisance but not a major economic factor to Hawaii’s papaya industry since the 1960s (3). This was due to the relocation of the papaya industry to Hawaii island in the Puna district which did not have PRSV. By the 1970s, Puna was producing 95% of the state’s papaya. However, PRSV was a real threat because the virus had established itself in back yards of homes in the town of Hilo which was only about 19 miles away from the Puna production area. Thus, research efforts to develop control measures for PRSV were initiated in 1978 starting with purification and characterization of PRSV, the development of a mild mutant, and the testing of cross protection. Research on transgenic papaya was started in 1985. The work on transgenic papaya up to 1998 has been presented in the APSnet Feature and other articles. This section provides only a brief summary.
Using the concept of pathogen-derived resistance, the coat protein gene of a mild mutant of a PRSV strain from Hawaii was used in biolistic transformation of embryogenic cultures of red-fleshed Sunset cultivar (2). We had not succeeded in obtaining a suitable transgenic papaya of the yellow fleshed Kapoho, which was by far the dominant cultivar growing in Hawaii. Transgenic line 55-1 of Sunset was inbred to homozygosity for the single copy coat protein gene and named SunUp. The Rainbow cultivar was developed to create a virus-resistant, transgenic yellow-fleshed papaya to replace virus-susceptible Kapoho. Rainbow is an F1 hybrid from SunUp and nontransgenic Kapoho (7).
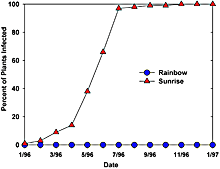
In 1995, a large field trial of Rainbow and SunUp was installed in a severely infected farm located in the Kapoho region of Puna where the virus was already widespread (1). A section of the trial included small replicated blocks of Rainbow, SunUp, nontransgenic Sunset, and a transgenic Sunset line 63-1. Adjacent to these replicated blocks was a large solid block of Rainbow that was surrounded by border rows of virus-susceptible Sunrise. PRSV inoculum source existed in a nearby infected block of nontransgenic Sunrise as well as selected Sunrise plants in the border rows that were mechanically inoculated with PRSV. The results were dramatic, all nontransgenic plants became infected within 11 months of starting the field trail while none of the transgenic test plants became infected (Fig. 2 and 3). This resistance was still evident up to termination of the trials in early 1998. The transgenic Rainbow yielded an estimated 125,000 lbs per acre per year, as compared to 5,000 lbs per acre per year for the nontransgenic Sunrise.
 |
|
 |
|
Fig. 2. Kapoho field trial started in 1995, showing a solid block of PRSV-resistant Rainbow growing well while the surrounding susceptible non-transgenic Sunrise is severely infected with PRSV. Picture taken 19 months after start of the field trial. |
|
Fig. 3. Comparative infection of transgenic and nontransgenic papaya in the 1995 field trial in Kapoho (see Fig. 2). Note that the transgenic Rainbow were resistant while all the nontransgenic Sunrise became infected within a year. |
| |

Fig. 4. Front cover of a brochure distributed during the celebration on the inaugural seed distribution of transgenic Rainbow and SunUp to growers on May 1, 1998.
|
Transgenic line, 55-1, the parent of SunUp and Rainbow, was deregulated (3) within two years after documents were submitted to the Animal Plant Health Inspection Service (APHIS), the Environmental Protection Agency (EPA), and the Food and Drug Administration (FDA). APHIS considered the impact on agricultural environments, EPA considered the pesticidal aspect of the viral coat protein produced by the transgenic papaya, and consultation was held with FDA which considered the food safety aspect of the transgenic papaya. Licenses that were needed to commercialize the transgenic papaya were obtained by April 1998. Seeds were distributed to growers on May 1, 1998 following a celebration held in Hilo, Hawaii (Fig. 4). Efforts to deregulate the transgenic papaya were carried out by the investigators and funding for deregulation and licensing was provided by the Papaya Administrative Committee (PAC), a grower organization regulated under a USDA marketing order. The transgenic papaya could now be used in efforts to translate the hope of controlling PRSV into a reality.
Reclamation of Infected Areas in Puna
Production of transgenic papaya seeds was started in 1996, two years before the transgenic papaya was commercialized. Seed production focused on generating large supplies of Rainbow because of its impressive performance in the 1995 field trial, high yields, excellent commercial characteristics, and the farmer and consumer preference for yellow-fleshed papaya. Funding to produce the seeds was obtained by PAC. Acquaintance with the papaya growers in the confined geographic area of PRSV damage allowed for a rather unique opportunity to document the adoption and reaction of a majority of the papaya farmers to the transgenic crop soon after the seeds were released. These aspects will be described in a future APSnet Feature.
In 1998, PRSV was widespread in Puna where numerous abandoned fields of PRSV-infected papaya dotted the landscape. Thus, it was assumed that many of the new plantings were going to be interspersed amongst infected fields, subjecting the transgenic papaya to inoculation pressure of PRSV. Although the field trials had showed that resistance held up under high disease pressure, we were somewhat anxious as large-scale plantings of transgenic papaya were started among the many infected papaya fields. However, observations soon showed that the resistance of transgenic papaya, which was nearly all Rainbow, held up under conditions of very high PRSV inoculation pressure.
The resistance of the transgenic papaya under rather strong disease pressure allowed farmers to directly reclaim their farms without first clearing their land of all infected papaya trees. Common scenarios were to grow transgenic plants next to mature papaya plants, which were subsequently cut after the transgenic plants were established (Fig. 5), or to simply grow transgenic papaya among abandoned fields (Fig. 6). In all cases, the transgenic papaya remained virus-free. Within a year after the release of the seeds, it was rather common to see many fields of healthy Rainbow (Fig. 7) and this scene is still predominant in 2004.
 |
|
 |
|
Fig. 5. PRSV infected papaya that were cut down in the foreground and healthy transgenic Rainbow papaya in the background. Resistance of Rainbow held up even under strong inoculum pressure of PRSV. Picture taken in 1999. |
|
Fig. 6. Green healthy transgenic Rainbow papaya growing among PRSV-infected trees in an abandoned papaya field. Picture taken in 1999. |
| |

Fig. 7. By 1999 healthy fields of transgenic papaya were commonly seen as opposed to the period of 1994-1998 where it was very difficult to find healthy papaya fields in Puna (see Fig. 1A). Picture taken in 1999. |
|
Impact of Transgenic Papaya
Controlling PRSV. The field resistance of the transgenic papaya in Puna proved to be durable, a result that was not necessarily predictable given that greenhouse tests had shown Rainbow to be resistant to the several PRSV isolates from Hawaii but susceptible to a range of isolates from outside of Hawaii (9). For example, isolates from Guam, Taiwan, and Thailand overcame the resistance of Rainbow. Unlike Rainbow, however, SunUp is resistant to many PRSV strains from regions outside of Hawaii. Recent studies in our laboratories and others have shown that the pathogen-derived resistance to plant viruses is due to the mechanism of post-transcriptional gene silencing or RNA-interference (8). A practical consequence is that increasing the transgene dosage can lead to increased resistance. Thus, transgene dosage is the likely reason that SunUp shows broader resistance than Rainbow, in that SunUp is homozygous for the inserted coat protein gene, while Rainbow is hemizygous (or has half the gene dosage of SunUp) because it is an F1 hybrid between SunUp and the nontransgenic Kapoho.
The continued popularity of Rainbow points out that factors other than resistance play a large role in the commercial adoption of the transgenic papaya. While it would seem prudent that SunUp should be the cultivar of choice for ensuring the resistance to PRSV in Hawaii, the demand for Rainbow has grown because the industry and market prefer that cultivar. Thus, Rainbow plantings are constantly being monitored for evidence of breakdown of resistance. Fortunately, in Puna and Oahu, the resistance has held up well under diverse conditions of plantings and disease pressure.
Increased production of papaya. The release of the transgenic papaya resulted in an increase of papaya production in Hawaii and Puna. The following observations were made for Puna up to the year 2002 (Table 1). In 1992, Puna produced 53 million of the state’s 55 million pounds of fresh papaya. The production remained high for two years following the discovery of PRSV in Puna due to massive efforts to control the spread of the virus. However, by 1995 papaya production in Puna had dropped to 39 million pounds and was down to 26 million pounds in 1998 when transgenic seeds of cultivars were released to farmers. Production of papaya in Puna increased starting in 2000 and peaked at 40 million pounds in 2001 with 35 million pounds being produced in 2002. The effect of PRSV on papaya production in Puna can also be seen by the drop in the total percentage of Hawaii’s fresh papaya production that was produced in Puna. In 1992, Puna accounted for 95% of the total production, but this figure subsequently dropped to 65% in 1999 and has since risen to 84% in 2002.
Table 1. Fresh papaya productiona in the state of Hawaii and in the Puna district from 1992-2002.
|
Year |
Fresh papaya utilization in Hawaii |
Total
(× 1,000 lbs) |
Puna
(× 1,000 lbs) |
% |
| (virus in Puna) 1992 |
55,800 |
53,010 |
95 |
| 1993 |
58,200 |
55,290 |
95 |
| 1994 |
56,200 |
55,525 |
99 |
| 1995 |
41,900 |
39,215 |
94 |
| 1996 |
37,800 |
34,195 |
90 |
| 1997 |
35,700 |
27,810 |
78 |
| (transgenic seeds released) 1998 |
35,600 |
26,750 |
75 |
| 1999 |
39,400 |
25,610 |
65 |
| 2000 |
50,250 |
33,950 |
68 |
| 2001 |
52,000 |
| 0 |
77 |
| 2002 |
42,700 |
35,880 |
84 |
a Data were compiled from USDA Statistical Reports of Papaya grown in Hawaii (www.nass.usda.gov/hi).
The impact of the transgenic papaya in increasing papaya production in Puna is also seen by analyzing the relative bearing acres of Rainbow and the nontransgenic Kapoho (Table 2). In 1998, Puna production was 26 million pounds from 1,640 acres of bearing Kapoho, since Rainbow had not yet produced mature fruit. In 2000, Puna production had increased to 34 million pounds from 1,190 bearing acres, with Kapoho comprising 32% and Rainbow comprising 50% of the acres. In 2001, 40 million pounds were produced from 1,675 bearing acres with Kapoho and Rainbow accounting for 39 and 41% of the acreage, respectively. In 2002, the bearing acreage dropped and the amount of Kapoho rose to 49% while Rainbow remained steady at 37%. Production dropped from 40 million pounds in 2001 to 36 million pounds in 2002. These data suggest that Rainbow accounts for at least half of the fresh fruit production in Puna. Furthermore, production of similar amounts of papaya can be obtained with less acreage. This latter observation is attributed to the higher level of production of Rainbow compared to nontransgenic Kapoho.
Table 2. Bearing acres in Puna of nontransgenic Kapoho and transgenic Rainbow and the relationship to production (× 1,000 lbs) of fresh fruit utilizeda.
| Year |
Bearing acres |
% Kapoho |
% Rainbow |
Production |
| 1998 |
1640 |
100 |
0 |
26,250 |
| 2000 |
1190 |
32 |
50 |
33,950 |
| 2001 |
1675 |
39 |
41 |
40,290 |
| 2002 |
1385 |
49 |
37 |
35,880 |
a Data were compiled from USDA Statistical Reports of Papaya grown in Hawaii (www.nass.usda.gov/hi).
Help in production of nontransgenic Kapoho in Puna. One might ask the logical question: Why doesn’t Hawaii produce only transgenic papaya? In fact, it is critical that Hawaii continues to produce nontransgenic papaya to supply the market in Japan, as will be discussed below. Arguably, one of the major contributions that the transgenic papaya has made to the papaya industry is that of helping in the economical production of nontransgenic papaya (4). This has occurred in several ways. First, the initial large-scale planting of transgenic papaya in established farms along with the elimination of abandoned virus-infected fields drastically reduced the amount of available virus inoculum. The reduction in virus inoculum allowed for strategic planting of nontransgenic papaya in areas that were free of infected plants and were not surrounded by areas of infected plants, such as had been present in 1992. In fact, the Hawaii Department of Agriculture (HDOA) instituted a plan in 1999 to ensure the production of nontransgenic papaya in the Kahuwai area of Puna by taking advantage of the natural reduction in inoculum pressure due to the large-scale plantings of Rainbow in Puna, the isolation of the proposed area from established papaya fields, and the fact that the prevailing winds in Kahuwai come from the ocean which borders the area (4). Furthermore, growers were to monitor for infection and rogue infected plants quickly. This program successfully helped growers who followed the recommended practices to economically produce Kapoho without major losses from PRSV.
Although definitive experiments have not been carried out, it seems that transgenic papaya can provide a buffer zone to protect nontransgenic papaya that are planted within the confines of the buffer. The reasoning is that viruliferous aphids will feed on transgenic plants and thus be purged of virus before traveling to the nontransgenic plantings within the buffer. This approach also has the advantage that it allows the grower to produce transgenic and nontransgenic papaya in relatively close proximity. Timely elimination of infected trees would need to be practiced to delay large-scale infection of the nontransgenic plants.
Expanding papaya production areas and the diversification of cultivars available for Hawaii. The availability of PRSV resistant papaya provided options for papaya growers on Oahu island. Prior to the release of transgenic papaya, Oahu growers farmed only small plots of papaya due to the effect of PRSV on production. Growers on Oahu enjoy a niche market, growing Rainbow papaya for residents in Honolulu and other urban areas of the island. The transgenic papaya has also allowed for the development of new cultivars to meet niche market needs on Oahu island. The new transgenic cultivar Laie Gold, which is a hybrid between Rainbow F2 and the nontransgenic Kamiya papaya also serves a niche market on Oahu island. Since Rainbow F2 is not homozygous for the coat protein gene, Laie Gold needs to be micropropagated to achieve uniformity of production. The micropropagation of the papaya also has the added benefits of ensuring the production of only hermaphrodite plants demanded by the market, earlier and lower bearing trees with initially higher yields, and providing selected, superior clones that could result in improved quality and yield.
Since the introduction of transgenic papaya, the number of cultivars available to papaya growers in Hawaii has actually increased. As noted earlier, Kapoho accounted for 95% of Hawaii’s papaya market in 1992. Now Rainbow and Kapoho are dominant and the transgenic SunUp, Laie Gold, and the nontransgenic Sunrise make up a small but significant part of the cultivars grown in Hawaii. In addition to the cultivars mentioned, newer ones have been developed for niche markets include large-fruited, firm cultivars used as green papaya in South and Southeast Asian cuisine and a red-fleshed Laie Gold progeny called Red Kamiya that is gaining in the Oahu market. In addition, initial hybrids that were made with Rainbow and nontransgenic Kamiya have been backcrossed four times to nontransgenic Kamiya. Selected lines will be field trialed in 2004 (M. Fitch, S. Ferreira, unpublished data).
Challenges Facing Hawaii’s Papaya Industry
Although a major constraint to papaya production in Hawaii was eliminated with the introduction of PRSV-resistant transgenic papaya, Hawaii’s papaya industry still faces a number of challenges. Some of these are penetrating the markets in Canada and Japan, growing nontransgenic papaya, and the durability of the resistance of transgenic papaya.
Canadian and Japanese markets. Japan and Canada are large markets for the Hawaii papaya industry. Currently, Japan accounts for 20% of Hawaii’s export market, while Canada accounts for 11%. Canada approved the import of SunUp and Rainbow transgenic papaya in January 2003, and transgenic papaya shipments are continuing to Canada. However, the sale of transgenic papaya in Japan has not yet been approved. Meanwhile, it is critical that papaya shipments to Japan are not contaminated with transgenic papaya. Several steps are being taken to minimize contamination.
At the request of Japanese importers, HDOA adopted an Identity Preservation Protocol that growers and shippers must adhere to in order to receive an Identification Preservation Protocol (IPP) certification letter from HDOA that accompanies the papaya shipment. This is a voluntary program. Papaya shipments with this certification are allowed to be distributed in Japan without delay during the time Japanese officials are doing spot testing to detect contaminating transgenic papaya. In contrast, papaya shipments without this certificate must remain in custody at the port of entry until Japanese officials complete their spot checks for transgenic papaya. Completing the tests may take several days or a week, during which time the fruit lose quality and marketability.
Some significant features of the Identity Preservation Protocol are that the nontransgenic papaya must be harvested from papaya orchards that have been approved by HDOA. To get approval, every tree in the proposed field must be initially tested for the transgenic reporter gene (1,3-β-glucoronidase) that is linked to the virus resistance gene, and found negative; the trees (nontransgenic) must be separated by at least a 15-foot papaya-free buffer zone; and new fields that are to be certified must be planted with papaya seeds that have been produced in approved non-GMO fields. Tests for detecting transgenic papaya trees in the fields are monitored by HDOA and conducted by the applicant who must submit detailed records to HDOA. Before final approval of a field, HDOA will randomly test one fruit from 1% of papaya trees in the field. If approved by HDOA, fruit from these fields can be harvested. Additionally, the applicant must submit the detailed protocols that the applicant will follow to minimize the chance of contamination of non-GMO papaya by GMO papaya. This includes a protocol by the applicant on the random testing of papaya before they are packed for shipment. If the procedures are followed and tests are negative, a letter from HDOA will accompany the shipment stating that the shipment is in compliance with a properly conducted Identity Preservation Protocol.
The above procedure represents a good faith effort by HDOA and applicants to prevent transgenic papaya contamination in shipments of nontransgenic papaya to Japan. It also illustrates meaningful collaboration between Japan and HDOA to continue shipments of nontransgenic papaya to Japan with a minimum of delay once they arrive in Japan, and yet adhere to the policy that transgenic papaya will not commercially enter Japan until it is deregulated by the Japanese government. These efforts, along with the effectiveness of the transgenic papaya in helping in the economic production of nontransgenic papaya, have allowed Hawaii to maintain significant shipments of the latter to Japan.
Obviously, deregulation of transgenic papaya in Japan will circumvent much of the concern of accidental introduction of transgenic papaya into Japan. To this end, efforts to allow the transgenic papaya into Japan were initiated by the PAC soon after the transgenic papaya was commercialized in Hawaii. Again, the researchers took the lead in developing the petition. Approval of the transgenic papaya in Japan requires the approval of the Ministry of Agriculture Fisheries and Forestry (MAFF) and the Ministry of Health Labor Welfare (MHLW). The petition to the MAFF was approved in December of 2000. The petition process for approval by MHLW is still in progress. An initial petition was submitted to MHLW in April 2003. MHLW requested more information, which is currently being generated by the researchers.
Achieving durable resistance. Finally, the issue of durability of resistance should be considered. Studies have shown that SunUp papaya has broader resistance than Rainbow, but the reality is that Rainbow is the dominant transgenic papaya grown in Hawaii. So far, we have not observed breakdown of resistance of Rainbow in Puna or on Oahu. However, we need to be on guard for this possibility. The likelihood of new virulent strains developing due to recombination of PRSV strains in Puna with the coat protein transgene of Rainbow is remote. A more realistic danger is through the introduction of PRSV strains from outside of Hawaii. We have shown that Rainbow or hemizygous 55-1 is susceptible to many strains of PRSV from outside of Hawaii, including strains from Guam, Taiwan, and Thailand. Goods imported to or in transit through Hawaii thereby increase the opportunity to also introduce new PRSV strains into Hawaii. Technically, SunUp should be resistant to many strains of PRSV that might be introduced into Hawaii. However, as noted above, the red-fleshed SunUp is not the preferred cultivar in Hawaii.
A potential solution is to develop transgenic Kapoho that is resistant to a wide range of strains. This could be used as a stand-alone cultivar, or it could serve as a transgenic parent for creating a new type of Rainbow by crossing the transgenic Kapoho with SunUp. This F1 hybrid should have the horticultural characteristics of current Rainbow but would likely have much wider resistance then the current Rainbow due to increase in coat protein gene dosage. We indeed have developed transgenic Kapoho that is resistant to a range of PRSV strains (D. Gonsalves, unpublished data). However, the time frame to commercialize this transgenic Kapoho may be longer than the time it took to commercialize line 55-1. These circumstances point to the fact that we need to carefully guard against the introduction of PRSV strains into Hawaii and to maximize the usefulness of our existing transgenic cultivars. Alternatively, we backcrossed Rainbow F2 plants with Kapoho four times to obtain backcrossed lines that were subsequently self-pollinated. Homozygous plants yielding BC4 fruit nearly identical to Kapoho were developed (M. Fitch, S. Ferreira, unpublished data).
Guard against large scale resurgence of PRSV in nontransgenic papaya in Puna. Despite the efforts to protect nontransgenic Kapoho from PRSV, observations suggest that PRSV infections are increasing in nontransgenic papaya in Puna (Fig. 8). As virus inoculum builds up in Puna, it will become more difficult to economically produce nontransgenic papaya. Strict attention needs to be paid to planting nontransgenic papaya in as much isolation as possible, doing timely elimination of infected trees, and to plowing under nontransgenic plantings that are no longer in production. The latter will reduce the amount of available PRSV inoculum.
| |

Fig. 8. PRSV is still in Puna, and more infections are occurring among the non-transgenic Kapoho. This site shows the dramatic difference between a block of nontransgenic papaya (foreground) adjacent to a block of transgenic Rainbow (background). Nontransgenic trees that became infected were cut down as seen in foreground. Plants were established at the same time. Picture taken in 2004. |
|
Transgenic Papaya as a Model for Technology Transfer
Since PRSV is a worldwide problem on papaya, which is widely grown in the tropics, other countries have showed interest in developing the technology for their use. Thus, a program was set up by one of the authors (Gonsalves) to develop and transfer the technology to interested countries. This section summarizes the status of the program and relates the progress to the current status of GMOs in general.
Starting in 1992, the technology transfer program has been implemented with agencies in the countries of Brazil, Jamaica, Venezuela, Thailand, and recently with Bangladesh and the east African countries of Tanzania, Uganda, and Kenya. Basically, it has involved students or scientists coming to the host institution (at that time, Cornell University) to develop a transgenic papaya that would be useful for their countries. Since the resistance of transgenic papaya can be narrow, the transgenic papaya was targeted for resistance to viral strains in their countries. The approach has been to utilize the coat protein gene from the country of origin and to transform papaya that are grown in that country. Technically, the project has progressed as planned. Transgenic papaya were taken to Jamaica, Brazil, and Thailand starting in 1996. In Brazil, the transgenic papaya has been subjected to a very limited field trial and is awaiting permission to be tested in large field trials. In Jamaica, the transgenic papaya has been tested in field trials, but deregulation efforts have stalled. In Thailand, the transgenic papaya has been field trialed extensively (Vilai Prasartsee, personal communication), two lines were selected for their horticultural characteristics and resistance, and the processes for deregulating the transgenic papaya are well under way and moving along. In Venezuela, a small field trial was attempted but activists destroyed it before useful data could be collected.
The technology transfer program has pointedly reaffirmed that it is relatively straightforward to develop transgenic papaya in a timely manner and to get it into the collaborating country. However, it appears that the processes for moving the product to commercialization have been vague and slower than the program that we followed in Hawaii. The efficiencies of the processes are likely due to the differing states of the target countries in their governmental programs for moving genetically engineered crops forward, relative to the U.S. system. Undoubtedly this is affected by the variations of acceptance of GMOs throughout the world.
Another approach we are taking is the development of transgenic papaya for Bangladesh. Unlike the other cases, the objective is to improve human health by making papaya readily available to the rural farmers or villagers who are often poor and show acute vitamin deficiency (especially vitamin A). By supplying them with seeds of transgenic papaya, they should be able to grow the plants in their back yards without the threat of PRSV. This project is in its initial phases. It is a bit different from the other technology transfer projects in that the goal is to develop the transgenic papaya as soon as possible in the U.S., and then send the transgenic papaya to Bangladesh, where the local authorities and scientists will usher the product through their system of testing and regulations. It is anticipated that the regulatory framework will be organized and coordinated while the transgenic papaya is being developed.
Summation
The 1998 APSnet Feature was entitled "Transgenic Papaya: A New Hope for Hawaii." It can be said that the transgenic papaya fulfilled the hope of the Hawaii papaya industry to control PRSV and to stabilize and restore the supply of papaya to nearly the level existing before PRSV entered Puna in 1992. There remain challenges to the Hawaii papaya industry, mainly in getting the transgenic papaya approved for sale in Japan. Due to its success, the transgenic papaya has often been referred to as the model for the use of biotechnology to help agriculture without investments by large companies. Indeed, transgenic papaya has been developed and transferred to other countries. In Jamaica, and especially in Thailand the transgenic papaya has performed very well under field trials, and deregulation procedures are progressing. However, it is very likely that the process will take much more time than it did in Hawaii. This is not due to the technical difficulties in product development, but is due to the GMO controversy. Thus, technology has moved along, and the major challenge will be to see how political processes proceed toward decisions on whether this technology will actually be used to fight this very severe problem in Thailand, Jamaica, and other countries.
Literature Cited
1. Ferreira, S. A., Pitz, K. Y., Manshardt, R., Zee, F., Fitch, M., and Gonsalves, D. 2002. Virus coat protein transgenic papaya provides practical control of papaya ringspot virus in Hawaii. Plant Dis. 86:101-105.
2. Fitch, M. M. M., Manshardt, R. M., Gonsalves, D., Slightom, J. L., and Sanford, J. C. 1992. Virus resistant papaya derived from tissues bombarded with the coat protein gene of papaya ringspot virus. BioTechnol. 10:1466-1472.
3. Gonsalves, D. 1998. Control of papaya ringspot virus in papaya: A case study. Ann. Rev. Phytopathol. 36:415-437.
4. Gonsalves, D., and S. Ferreira. 2003. Transgenic papaya: A case for managing risks of papaya ringspot virus in Hawaii. Online. Plant Health Progress doi:10.1094/PHP-2003-1113-03-RV.
5. Gonsalves, D., Ferreira, S., Manshardt, R., Fitch, M., and Slightom, J. 1998. Transgenic virus resistant papaya: New hope for control of papaya ringspot virus in Hawaii. APSnet Feature, American Pythopathological Society.
6. Lius, S., Manshardt, R. M., Fitch, M. M. M., Slightom, J. L., Sanford, J. C., and Gonsalves, D. 1997. Pathogen-derived resistance provides papaya with effective protection against papaya ringspot virus. Mol. Breeding 3:161-168.
7. Manshardt, R. M. 1998. 'UH Rainbow' papaya. University of Hawaii College of Tropical Agriculture and Human Resources New Plants for Hawaii-1:2pp.
8. Tennant, P., G. Fermin, M. M. Fitch, R. M. Manshardt, J. L. Slightom, and D. Gonsalves. 2001. Papaya ringspot virus resistance of transgenic Rainbow and SunUp is affected by gene dosage, plant development, and coat protein homology. Euro. J. Plant Pathol. 107:645-653.
9. Tennant, P. F., C. Gonsalves, K. S. Ling, M. Fitch, R. Manshardt, Slightom, L. J, and D. Gonsalves. 1994. Differential protection against papaya ringspot virus isolates in coat protein gene transgenic papaya and classically cross-protected papaya. Phytopathology 84:1359-1366.
