C. B. Yandoc-Ables
E. N. RosskopfUSDA-ARS, Fort Pierce, FL
R. Charudattan
Plant Pathology Department,
University of Florida, Gainesville, FL
(Corresponding author: erosskopf@ushrl.ars.usda.gov)
Yandco-Ables, C.B., Rosskopf, E.N., and Charudattan, R. 2006. Plant Pathogens at Work. Progress and Possibilities for Weed Ciocontro Part 2: Improving Weed Control Efficiency. Online. APSnet Features. doi: 10.1094/APSnetFeature-2006-0906
See also Part 1
In Part 1 of this article, an introduction to biological control of weeds plant pathogens was provided. Research in this area is conducted using either the classical or the bioherbicidal approach. In the classical approach, a pathogen is imported from a foreign location to control an introduced weed. In the bioherbicide approach, an indigenous pathogen is used in an inundative application. In Part 2 of this article, research aimed at improving weed control efficacy for both approaches is discussed.
Enhancing Bioherbicides
Research on the development of plant pathogens for biological control using the inundative or bioherbicide approach has moved from determining host range and demonstrating pathogenicity to investigating systems that enhance the efficacy of these agents.
Formulation with water-retaining additives to reduce dew dependence. The requirement for long periods of dew or moisture is a major hurdle in the development of foliar fungal pathogens into bioherbicides, mainly because humidity and dew period duration can significantly limit disease initiation and disease progress. The utilization of formulations that minimize the influence of humidity is one approach to overcoming this restraint (6). The addition of an invert oil emulsion to conidial suspensions of Colletotrichum truncatum, a biocontrol agent for hemp sesbania (Sesbania exaltata), reportedly resulted in a 100% control of hemp sesbania in the absence of dew in the greenhouse. In the field, the same formulation caused >95% control of hemp sesbania, a similar level of control achieved with the chemical herbicide acifluorfen (18) (Fig. 1). Improved bioherbicidal efficacy by the addition of oil emulsions, with no or little exposure to dew, was also reported for Alternaria helianthi, a biocontrol agent for common cocklebur (Xanthium strumarium) (1); Alternaria eichhorniae for waterhyacinth management (95); Colletotrichum orbiculare, a biocontrol agent for Bathurst burr (Xanthium spinosum) (5); Bipolaris sacchari, a biocontrol agent for cogongrass (Imperata cylindrica) (113); and Drechslera gigantea, Exserohilum longirostratum, and E. rostatum, biocontrol agents against several grass weeds of citrus (32). Different modes of action have been proposed for the activity seen with invert emulsions, including phytotoxicity (e.g., 5,94) (Fig. 2).
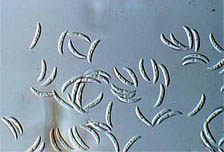
A |
|

B |
Fig. 1. (A) Spores of Colletotrichum truncatum, a biological control agent, (B) giving excellent control of hemp sesbania (Sesbania exaltata) in the field. Photos courtesy of Doug Boyette.
| |
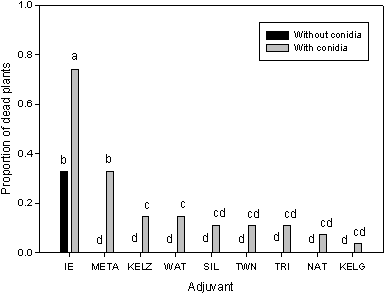
Fig. 2. Effect of various adjuvants on the bioherbicidal efficacy of Phomopsis amaranthicola. The adjuvants tested were: IE=invert emulsion, META=Metamucil, KEL=Kelzan, WAT=water, SIL=Silwet L-77, TWN= Tween 20, TRI=Triton X-100, NAT=Natrasol, and KELG=Kelgin. Efficacy was measured in terms of proportion of dead Amaranthus hybridus plants. Graph shows data from three trials (gray bars show the effect of conidia + adjuvant and black bars show the effect of the adjuvant alone). Bars with the same letters are not significantly different from each other at P = 0.05, as determined by Duncan's Multiple Range test. From Rosskopf et al (94). |
|
Broadening the spectrum of bioherbicides. While the specificity of bioherbicides is desirable in some situations, particularly in areas where crop and weed species are closely related, there are situations where several species of problematic weeds occur and a broad-spectrum weed control is required. This has been often cited as a major limitation to the bioherbicide approach in cropping systems (e.g., 56). Two approaches to broaden the host range of bioherbicides have been explored; the first one is via formulation, and the other is by combining pathogens.
Formulation with fruit pectin and plant filtrates allowed Alternaria crassa, a biocontrol agent specific to jimsonweed (Datura stramonium), to infect hemp sesbania, showy crotolaria, and eastern black nightshade, which were resistant to the fungus when applied without the fruit pectin and plant extracts (17). The selectivity of A. crassa and A. cassiae was also suppressed by formulation with an invert emulsion, resulting in the successful infection of eight other plant species other than their target hosts (3). In addition, C. gloeosporiodes, isolated from coffee senna (Senna occidentalis), controls sicklepod (S. obtusifolia) when formulated as invert emulsion or corn oil/Silwet L-77 emulsion (Doug Boyette, personal communication (Fig. 3)].
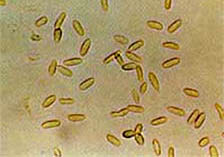
A |
|

B |
Fig. 3. (A) Spores of the biological control agent, Colletotrichum gloeosporioides, which was isolated from coffee senna (Senna occidentalis). (B) When combined with an oil-based formulation, the pathogen will also control sicklepod (S. obtusifolia). Photos courtesy of Doug Boyette.
Boyette et al. (21) were able to simultaneously control northern jointvetch (Aeshynomene virginica) and winged primrose (Jussiaea decurrens) by applying a combination of two host-specific pathogens, Colletotrichum gloeosporioides f. sp. aeschynomene and C. gloeosporioides f. sp. jussiae. Chandramohan and Charudattan (33) also demonstrated that a mixture of inoculum of three host-specific pathogens, namely Alternaria cassiae (specific to sicklepod), Phomopsis amaranthicola (specific to pigweeds), and Colletotrichum dematium f. sp. crotalariae (specific to showy crotolaria), resulted in the simultaneous and efficacious control of pigweed, sicklepod and showy crotolaria. Excellent control of seven weedy grass species (southern sandbur, large crabgrass, crowfootgrass, guineagrass, Texas panicum, yellow foxtail, and johnsongrass) was also achieved when conidia of three host-specific pathogens (D. gigantea, E. rostratum, and E. longirostratum) were applied together (Fig. 4) (34).
A different approach is to utilize pathogens that have a host-range that is not as restricted. One such example is the pathogen Myrothecium verrucaria. This pathogen was first evaluated for sicklepod (109) and kudzu control (22), but has since been evaluated for multiple weed targets (Fig. 5). Anderson and Hallett (4) found that culture filtrates from this fungus had broad-spectrum activity across many plant families. However, the use of M. verrucaria is likely to be limited due to its production of trichothecenes, although these toxins have not been detected in treated plants (2). Additional research into the necessity of trichothecenes for pathogenesis and their potential for mammalian toxicity when the pathogen is used in a bioherbicide system is warranted.
 |
|
 |
|
Fig. 4. The multiple-pathogen strategy proposed by Chandramohan et al (34) allows for the control of a broader spectrum of weeds than a single pathogen used alone and may address long-term concerns about the potential for resistance. |
|
Fig. 5. Impact of Myrothecium verrucaria on a mixed stand of spurge and purslane species. The broad-spectrum pathogen had no affect on tomatoes later transplanted into the plots (Doug Boyette, personal communication). Photo courtesy of D. Boyette. |
Enhancing bioherbicidal efficacy through delivery or application systems. Application of high volumes of bioherbicides (spore suspensions) has been done to ensure complete coverage of infection courts and to provide ample moisture needed for spore germination for maximum infection (70). The requirement for high application volumes for bioherbicides can deter their use because high volumes entail transport of a heavier load to the application site and longer application times. It has been shown that high volumes do not necessarily ensure high levels of disease. According to Lawrie et al. (74), the number of lesions produced or the infection density is influenced not by the application volume alone but by the spray droplet size, droplet retention and distribution, inoculum concentration and spray application volume. The type of spray equipment employed, as well as the formulation and the inoculum concentration influences droplet size, droplet retention, and distribution.
Chapple and Bateman (35) reported significant differences in the number of deposited droplets containing the active ingredient (i.e., spores) and the number of deposited droplets that did not contain any spores in a spray equipment comparison. The percentage of droplets with more than one spore was 18.9% using the hydraulic flat fan, 0.5% using the air blast sprayer, and 81.5% using the spinning disc. The percentage of droplets without any spores was 67.3% for the hydraulic flat fan, 95% for the air blast sprayer, and 6.5% for the spinning disc. This study clearly indicates how the type of sprayer can reduce or improve bioherbicidal efficacy. Yandoc (113) employed two different sprayers [air assisted sprayer vs. hand-held ultra low volume sprayer (ULVA)] to apply Bipolaris sacchari conidia (formulated in an oil emulsion) to cogongrass (I. cylindrica) in the field and found that application with the ULVA resulted in greater damage (>50%) than with the air-assisted sprayer (<35%).
Another interesting approach to application technology involves the use of the virus Tobacco mild green mosaic virus (TMGMV) for the control of tropical soda apple (Solanum viarum) (41). This weed is a problem in cattle pastures, and control with a biological control agent requires applications over large acreages. The virus has a dramatic impact, causing complete plant death (Fig. 6). Novel application methods have been effective and could easily be implemented by ranchers. These include low pressure application (20 psi) of the virus combined with plant abrasion using either a section of chain-link fence or carpet, and high pressure application (400 psi) directly to plants (Fig. 7).
| |

Fig. 6. Impact of the application of the biological control agent, Tobacco Mild Green Mosaic Virus (TMGMV), on tropical soda apple (Solanum viarum). |
|
| |

Fig. 7. Tropical soda apple is a problem weed on cattle ranches and its control requires coverage of large acreages. The use of the biological control agent, Tobacco Mild Green Mosaic Virus (TMGMV), is feasible through the use of novel application approaches, including abrasion of plants while spraying the virus and high-pressure spraying but not with the mow-and-spray application. |
|
Other ways of delivering bioherbicides, specifically solid-based formulations, have been tested for pre-emergence bioherbicides that are meant to attack weeds at or below the soil surface (19). Among the substrates used are Pesta (wheat-gluten matrix) for
C. truncatum,
A. crassa, and
F. lateritium (42) and
Fusarium oxysporum f.sp.
orthoceras (96), and cornmeal-sand for
F. solani f. sp.
cucurbitae, a bioherbicide for Texas gourd (20). Formulation with solid substrates allows for improved shelf life and also acts as a buffer when extreme conditions in the field occur (15).
Hutchinson (64) tested the strategy of enhancing bioherbicidal efficacy of Trichoderma virens by applying it with composted chicken manure. This study demonstrated that composted chicken manure supported the growth of T. virens as well as its production of viridiol, an antibiotic compound that can inhibit weed seed germination and emergence. Composted chicken manure is believed to provide the nutrient base necessary for T. virens to produce viridiol. In greenhouse tests, the application of T. virens infested composted chicken manure reduced the emergence by 77% and dry weight by 68% of naturally occurring miscellaneous weed species (Amaranthus retroflexus, Portulaca oleracea, Mollugo verticillata, Abutilon theophrasti, and Echinochloa crus-galli) (64). Similarly, Heraux et al. (59) used the combination of an allelopathic cover crop, rye, with the Trichoderma-inoculated compost to control weeds in transplanted vegetables.
Enhancing biocontrol efficacy through selection and use of amino acid excreting strains. Tiourebaev et al. (103) have attempted a novel approach to enhancing virulence of weed biological control agents by selecting for strains that are capable of excreting high levels of amino acids that can suppress the growth and development of plants. Tiourebaev et al. (103) reported improved efficacy of Fusarium oxysporum f.sp. cannabis Cs95, a potential biocontrol agent for Cannabis sativa. Valine-excreting mutants caused greater control (70 to 90%) of C. sativa, as compared to 25% control by the wild type. The mutants also caused severe wilting within a shorter period (2 to 3 weeks) after application, as compared to the wild type, which caused severe wilt and death after 6-8 weeks. Test plants inoculated with the valine-excreting mutants also caused leaf distortion, loss of stem apical dominance and stunted growth. The mutants failed to infect or cause damage to other plant species tested, indicating no change in the host-range. Other weed-pathosystems that might employ this approach are under investigation (108,116)
Combination of biocontrol agents with herbicides and other chemical agents that predispose weeds to infection. One of the many factors that can influence the level of weed suppression through biological means is the ability of a target weed to resist infection and colonization by the biological control agent. Hoagland (61) observed that the application of the biocontrol agent Alternaria cassiae to its target weed, sicklepod (Cassiae obtusifolia), resulted in increased activity of phenylalanine ammonia-lyase (PAL), an enzyme responsible for the synthesis of phenolic compounds that have been shown to protect plants from pathogen attack. Hoagland (62) presented several approaches for improving biocontrol efficacy by disrupting the target weed’s defense mechanisms, including the use of herbicides or other compounds that affect key enzymes, blocking the synthesis of secondary plant metabolites, or breaking down physical barriers to pathogen attack (e.g., epicuticular waxes), all of which have been tried with encouraging results (53,60,63,87).
Hirase et al. (60) observed enhanced bioherbicidal efficacy of Exserohilum monoceras on Echinochloa crus-galli, a weed in paddy rice (Oryza sativa L.), when it was applied with δ-aminolevulinic acid, a five-carbon amino acid and precursor of tetrapyroles, which are involved in the bleaching and killing of plant tissue. Gressel et al. (53) also demonstrated that the efficacy of a weakly pathogenic biological agent, C. coccodes on velvetleaf (Abutilon theophrasti), can be improved by applying chemical agents that repress host plant defenses, particularly the formation of callose. In their study, a low dose of fungal inoculum applied with calcium chelators resulted in increased infectivity by C. coccodes and reduced callose formation. Hodgson et al. (63) reported 75-90% velvetleaf mortality from the application of tank-mix combinations of C. coccodes and lower rates of thidiazuron, a plant growth regulator. Tank mixes of C. coccodes and thidiazuron were almost twice as effective as the same rates of thidiazuron applied alone in killing velvetleaf or reducing stand density at harvest (63).
The strategy of applying sub-lethal rates of the herbicide glyphosate with conidia of the biocontrol agent Alternaria cassiae to improve the level of weed control was also tested by Sharon et al. (97) and Peng and Byer (87). Sharon et al. (97) demonstrated that the ability of glyphosate to inhibit the accumulation of phytoalexin rendered sicklepod seedlings more susceptible to infection by A. cassiae even at low inoculum concentration levels. Peng and Byer (87) reported greater suppression of green foxtail (Setaria viridis) with a mixture of low rates of sethoxydim (one tenth of recommended rates) and Pyricularia setariae as compared to the level of control achieved with herbicide or the pathogen alone. Several other pathogens have been tested for their compatibility with other crop protection chemicals, including herbicides with which they might be tank-mixed (112,115) (Fig. 8). Cook et al. (44) have developed a system to integrate low doses of herbicide (glyphosate), a foliar desiccant (ammonium sulfate), and Alternaria destruens to control Cuscuta pentagona, demonstrating a method to obtain effective integrated control of this difficult-to-control parasitic weed.
| |
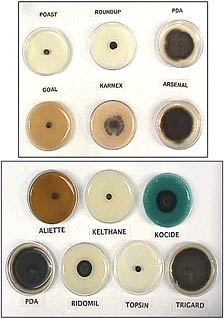
Fig. 8. Fungi with potential for use as biological weed control agents in agro-ecosystems should be tested for compatibility with other pesticides that may be used in the system. Pictured here is a Petri plate assay of Dactylaria higginsii, a potential biological control agent for nutsedges, grown on media amended with pesticides that might be used in a conventional tomato production system (115). |
|
Techniques for the Improvement of Weed Control Efficacy with Pathogens
Weed control through pathogen application and plant competition. The negative impacts of plant disease on plant growth and development have been shown to inhibit the target weed’s ability to compete with non-target plants. An early example of this phenomenon with classical biological control is Puccinia chondrillina, a rust specific to skeletonweed (Chondrillina juncea), which inhibited that plant’s ability to compete with subterranean clover (Trifolium subteraneum) (54). Similarly, Puccinia lagenophorae, a rust pathogen specific to common groundsel (Senecio vulgaris), reduced that plant’s ability to compete with lettuce, resulting in higher lettuce yields (86). Velvetleaf growth was stunted and seed production was reduced by the presence of C. coccodes, rendering it less competitive with the soybean crop (48). Dactylaria higginsii applied to mixed plantings of tomato and purple nutsedge reduced interference from this weed and increased the yield of tomato (67).
Application of Bipolaris sacchari to mixed plantings of cogongrass (Imperata cylindrica) and bahiagrass (Paspalum notatum) in greenhouse studies resulted in reduced cogongrass growth (reduced plant height, number of rhizomes produced, and aboveground biomass) and allowed bahiagrass to flourish (114). This strategy involves the specific suppression of a weedy grass species while allowing for a beneficial grass species to establish and take over the niche vacated by the weed and prevent its re-establishment (Fig. 9). Control of hedge bindweed (Calystegia sepium) with the application of Stagonospora convolvuli strain LA39 in combination with a red clover (Trifolium pretense) cover crop was evaluated by Guntli et al (55) in the greenhouse and the field. In the greenhouse, the reduction in the biomass of hedge bindweed was attributed to the cover crop since the infection level by S. convolvuli was not sufficient to cause significant damage. However, in the field, the fungus significantly reduced the ground area covered by hedge bindweed due to high disease level and spread of disease by rain splash, but the combined effects of the fungus and the cover crop was not observed due to poor growth of red clover. This study indicated the good potential of this strategy if a more competitive cover crop was used, such as Stellaria media (55).

A |
|

B |
Fig. 9. (A) Selective control of cogongrass (Imperata cylindrica) by Bipolaris sacchari, formulated in an invert emulsion, in a mixed planting of the weed in bahiagrass (Paspalum notatum). (B) Control of cogongrass, as seen in the pot on the left side, allows bahiagrass to flourish when compared to the uninoculated control on the right.
Although it would seem logical that weed interference could be reduced by competition from increased crop plant density coupled with bioherbicide applications, Pitelli et al. (88) did not find evidence to support this hypothesis. In their study, soybean planting density did not affect the mortality or the dry weight of sicklepod (Senna obtusifolia) sprayed with Alternaria cassiae and/or Pseudocercospora nigricans. While similar studies of crop-weed-bioherbicide combinations are not common, the need for this type of competition study is necessary to characterize possible avenues for enhanced control using integrated weed management systems that include bioherbicides.
Biocontrol and host plant resistance. A novel method of utilizing non-target plant competition was tested recently by Marley et al. (79) for management of striga (Striga hermonthica). Fusarium oxysporum strain PSM 197 (grown on gritted sorghum grain) was applied in each planting hole before sowing seeds of the resistant sorghum cultivar Samsorg 40 (ICSV 111) and the tolerant landrace Yar’ruruka. It was observed that plots treated with F. oxysporum and planted to each of the cultivars had significantly higher crop yield and lower striga infestation as compared to plots that were planted with the same cultivars but not treated with F. oxysporum. This resulted in increased profits for farmers in the Nigerian savanna.
Deleterious rhizobacteria and cover crops for improved weed control. The potential of deleterious rhizobacteria (DRB) to serve as control agents of weeds relies on their ability to reduce weed emergence and to delay weed growth and development, thereby giving the crop a competitive advantage (15). Reduced downy brome (Bromus tectorum) population and 18-35% yield increase in winter wheat through the application of the DRB Pseudomonas fluorescens strain D7 has been reported by Johnson et al. (66) and Kennedy et al. (68). However, there are also reports of inconsistent performance by DRB, due to poor survival or low activity once applied in the field. Li and Kremer (76) have tested an integrated approach utilizing DRB and cover crops for improved weed control. In this approach, the cover crops would support the proliferation and activity of the DRB before weed seed germination occurred, which enhancs the level of weed suppression by the cover crops. The DRB-cover crop combination caused greater weed biomass reduction as compared to cover crops alone. The characterization of cropping systems that support microbial populations that are weed-suppressive is an emerging area of research that shows great promise (47).
Combining pathogens and insects. Management of leafy spurge (Euphorbia esula-virgata) in natural areas has been problematic because chemical herbicides have been ineffective or inappropriate for most infested areas and leafy spurge can easily overcome damage from mechanical weed control measures and insect biocontrol agents (78). While the release of root-feeding flea beetles (Aphthona spp.) to control leafy spurge has resulted in successful insect establishment, the insects did not significantly reduce the leafy spurge population (27). However, a report by Caesar (28) about the frequent presence of soil-borne pathogens at release sites where leafy spurge is in decline has spurred interest in combining soil-borne pathogens (e.g., Rhizoctonia, Fusarium spp. and DRB isolated from leafy spurge roots) and flea beetles for a more effective control of leafy spurge (Fig. 10). This approach may also be possible for the control of white top (Cardaria draba), as plants with insect damage also harbor pathogens that are contributing to the decline of plant stands (Anthony Caesar, personal communication) (Fig. 11). Interestingly, these interactions are even more complex than they initially appear. Pathogens associated with insect-infested weeds have been found to be more highly virulent than the same species of fungi isolated from diseased weeds that are not infested with the insects (30,73). A three-way interaction has also been found among deleterious rhizobacteria, fungal plant pathogens and insects (73).

A |
|

B |
| |

C |
|
Fig. 10. (A) Field location with a significant leafy spurge (Euphorbia esula) population prior to the release of the flea beetle Aphthona flava and (B) four years after the release. (Photo credit Norman Rees, ARS, courtesy of Tony Caesar). (C) At several sites where the weed stand was reduced, soilborne pathogens were found to be associated with the affected plants. Photo courtesy of Tony Caesar.
| |

A |
|
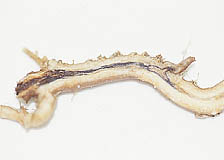
B |
|
| |
Fig. 11. (A) White top (Cardaria draba) with insect damage on foliage has (B) necrotic root systems associated with soilborne plant pathogenic fungi. Photos courtesy of Tony Caesar. |
|
The strategy for controlling weeds using the combination of insects and plant pathogens is supported by reports of population regulation resulting from damage from natural enemies (insects and plant pathogens) (36,40). The rust
Puccinia psidi is assumed to be an accidental introduction to Florida, where it was first found in the 1970s. It is currently under investigation as a biological control agent for Australian melaleuca (
Melaleuca quinquenervia) (90). Researchers in this area (Min Rayamajhi,
personal communication) reported significant reduction in the absolute density and diameter based density of the Australian melaleuca in the presence of and during an increase in the population of natural enemies (i.e., weevils, rust, lobate lac scales, psyllids and sooty molds) (Fig. 12). The impact of these pests is dramatic and allows for the regeneration of the understory (Fig. 13). The elucidation of the interactions between these natural enemies is the current area of research (91).
| |

A |
|

B |
|
| |
Fig. 12. (A) Early symptoms of rust (Puccinia psidii) and melaleuca psyllid (Boreioglycaspis melaleucae) damage on young melaleuca (Melaleuca quinquenervia) twigs. (B) Melaleuca twig defoliation and dieback caused by the rust, psyllids, and weevil (Oxyops vitiosa). Photos courtesy of Min Rayamajhi. |
|
| |

A |
|

B |
|
| |

C |
|
Fig. 13. (A) Crown thinning of Melaleuca quinquenervia resulting from multiple biological control agents, including rust, psyllids, and weevils. (B) and (C) Melaleuca crown thinning and tree mortality allowed development of understory vegetation. Photos courtesy of Min Rayamajhi.
The Future
The effort to develop plant pathogens as commercially-available biological weed control agents (bioherbicides) has not yielded the large number of products that the research would indicate could be available. However, research in this area has nonetheless contributed significantly to the science and technology of biological control. The development of this weed-control technology, albeit used to a very limited extent, has resulted in several commercial products, including four pathogens registered within the past five years. Furthermore, as Hallett (56) points out, there are opportunities for the development of bioherbicides for some specialized niches, such as parasitic, urban, and allergenic weeds. For example, Smolder (Alternaria destruens), has been registered recently for the control of several Cuscuta (dodder) species.
It is true that with the current state-of-the-art it is difficult for companies to sustain business with a single or few registered bioherbicide products. However, it is not a lack of proven efficacy that has limited the availability of these materials; it is the market-driven return on investment that is the constraint. For example, both DeVine and Collego are extremely effective materials, but according to Dave Goulet of Encore Technologies (personal communication), the market was too small and sporadic to maintain the economic viability of the products for his company. Fortunately, a new registrant, ARI Incorporated, has taken on the marketing of Colletotrichum gloeosporioides f. sp. aeschynomene (Collego pathogen), as the commercial product LockDown for use in rice in Arkansas, Louisiana, and Mississippi (Dave TeBeest, personal communication). In the developed world, the goal of most single pathogen-single weed bioherbicide research projects is a saleable product. Therefore, the long-term success of bioherbicides may depend on wholesale changes in consumerism and production systems that demand non-chemical approaches. Differences in regulatory requirements between countries may allow for some systems to gain greater acceptability in places where local production of inoculum for immediate use is realistic. In short, there are still stakeholders that would benefit from the bioherbicide technology (39).
Research on weed-pathogen systems has greatly contributed to knowledge in plant disease epidemiology, plant-microbe interactions, and biodiversity. These are all contributions that cannot be overlooked. This knowledge now provides the basis to understand and study multi-trophic interactions in weed-pathogen systems. Many of the problematic weeds are often found in monocultured crops or they exist in crops where low-cost weed control is critical. Although most biological control agents are too host-specific to individually address mixed weed populations in agronomic field crops, they can be targeted to manage those weeds that have the most impact on crop yield in high-value crops where control options are limited. Some cropping systems will inherently favor a single dominant weed species, leading essentially to a monoculture in which a host-specific biological control agent is ideally suited. Organic and conventional vegetable and herb production systems are particularly well suited to this approach and are in dire need of weed control options. Continuing to place emphasis on the development of integrated weed control systems that employ plant pathogens as components could greatly increase the number of successful biological weed control programs.
Disclaimer
Mention of a trademark, warranty, proprietary product or vendor does not constitute a guarantee by the United States Department of Agriculture and does not imply its approval to the exclusion of other products or vendors that may also be suitable.
Additional Resources
Weeds Newsletters
from Manaaki Whenua - Landcare Research
Classical Biological Control of Weeds
from Lethbridge Research Centre, Agriculture & Agri-Food Canada
TEAM Leafy Spurge
from USDA-ARS, Northern Plains Agricultural Research Laboratory
Invasive Plant Research Laboratory
from USDA-ARS
Agricultural Permits: Weed Biocontrol
from USDA, APHIS, PPQ
International Survey of Herbicide-Resistant Weeds
from WeedScience.org
Multistate Research Project S-1001: Development of Plant Pathogens as Bioherbicides for Weed Control from Botany & Plant Pathology, Purdue University
Literature Cited
See Literature Cited page.
