Anthuriums are perennial, herbaceous epiphytes with a climbing habit. They are produced commercially for the wide variety of colors and shapes of their heart-shaped spathes, often referred to as the floral portion. A long spadix projecting from the spathe bears the true flowers and seeds (Fig. 1). Heart-shaped leaves with one main midvein and several lateral veins are attached to long petioles.
| |

Fig. 1. Typical anthurium cultivar commercially produced for cut-flowers and flowering potted plants. Photo by A. R. Kuehnle. |
|
Anthuriums are in the family Araceae, which has over 100 genera including Anthurium, Dieffenbachia, Xanthosoma, Spathiphyllum, Epipremnum, Aglaonema, and Philodendron. The genus Anthurium encompasses over 1,500 species, more than 600 of which originate from Tropical America (23,27). The native habitat includes high elevation areas of Central America, Venezuela, Colombia, Ecuador, and Peru. The best known species is Anthurium andraeanum, which was discovered by Eduard André in 1870 during travels to Colombia and Ecuador (58). In the natural state A. andraeanum is epiphytic and can be found in mountain forests at elevations of 2400 ft where the spathes of this species are orange-red and blistered. Many of today’s cultivated anthuriums are named A. andraeanum but differ in appearance from the native species. The spathes may be smooth or blistered to varying degrees, and are available in a wide range of colors (27).
Introduction of A. andraeanum to Hawaii from London in 1889 was by S. M. Damon, who described it as having a spathe with shell-pink color (41). Plants were grown on the Damon estates at Moanalua, and from there it was slowly distributed to other growers via vegetative propagation. In the late 1930s and 1940s, growers in Hawaii learned how to propagate anthurium by seed, leading to increased cultivation and variation (26). The assortment available in the current market (Figs. 2 through 5) is a result of crosses between A. andraeanum cultivars, as well as crosses between A. andraeanum and other species, such as A. antioquiense (26,58).
 |
|
 |
|
Fig. 2. Anthurium andraeanum cultivar ‘Ozaki’. Photo by R. Cabos. |
|
Fig. 3. Anthurium andraeanum cultivar ‘Marian Seefurth’. Photo by T. Vowell. |
| |
 |
|
 |
|
| |
Fig. 4. Anthurium antioquiense cultivar ‘Pink Frost’. Photo by P. Toves. |
|
Fig. 5. Anthurium antioquiense cultivar ‘Tropic Fire’. Photo by P. Toves. |
|
Development of the Anthurium Industry in Hawaii
Anthuriums are the most important cut flowers in the Hawaiian floriculture industry today. This industry developed initially from hobbyists and backyard growers who supplied flower shops in Hawaii during the 1940s. Commercial operations for anthurium production in 1959 included 266 farms from Hawaii, 88 from Oahu, 7 on Kauai, and 4 on Maui (25). Increased worldwide demand for anthurium cut flowers in the 1970s boosted sales and increased production area from 40 acres to 400 acres in 1979. The industry reached its peak in 1980, supplying local, national, and international markets with up to 232,000 dozen flowers per month (25). Although yield was at 2.5 million dozen flowers in 1980, supply was insufficient to meet demand (26).
Large scale business operations in saran shadehouses (Fig. 6) were successfully exporting anthuriums to the U. S. mainland and Japan when the first outbreaks of anthurium blight were reported. Since then, production figures have decreased by 76% (57). Hawaiian production for 2004 was estimated at 617 thousand dozens. Hawaii’s floriculture industry was valued at $94.5 million in 2004 (57). Cut flowers sales were valued at $13.1 million, with anthuriums ranking as the top seller at $4.7 million.
| |

Fig. 6. Cultivation in saran-cloth shadehouse. Photo by D. Norman. |
|
The anthurium research program, initiated in Hawaii in 1950 by Dr. Haruyuki Kamemoto, led to the development of a breeding program for the commercial development and release of anthuriums to growers (30). The development of additional anthurium cultivars for the cut flower industry by breeders in Hawaii and the Netherlands has led to the availability of an assortment of varieties, with red and orange having most importance, followed by other colors such as salmon, cherry, and pink (30,58).
The top four producers of anthurium cut flowers worldwide are Netherlands, Hawaii, Mauritius, and Jamaica, followed by smaller tropical flower producers in the Philippines, Tahiti, Thailand, Malaysia, India, Brazil, Trinidad, Guadeloupe, Martinique, Florida and California (11,39,54,55). Although anthuriums were initially grown for cut flowers, the industry has since expanded into commercialization of potted plants. A. scherzerianum cultivars are the main potted anthuriums in Europe. In Hawaii, anthurium cultivars generally are hybrids of A. andraeanum and/or A. antioquiense, with varieties based on characteristics of the colorful spathe and upright spadix (Fig. 7).
| |

Fig. 7. Anthurium antioquiense cultivars have a small heart-shaped spathe and upright spadix. Photo by A. R. Kuehnle. |
|
Introduction of Bacterial Blight into Hawaii
Anthuriums were relatively insect and disease free in the early years of production, and commercial plantings established in Hawaii in the 1940s were successfully grown for forty years. Disease problems, including anthracnose, root rot, and lesion nematode, developed as cultivation increased from small plots to large operations. The most serious disease problem to strike the industry is bacterial blight caused by the pathogen Xanthomonas axonopodis pv. dieffenbachiae (previously, X. campestris pv. dieffenbachiae) (43). The disease was first reported in Kauai in 1972 (21) but had little impact on the industry until 1981, when plants began to die in large numbers on farms in Hilo (42). The disease reached epidemic proportions in 1985-1989, destroying the production of approximately 200 small farms (Fig. 8). Hawaii’s production dropped from a record high of approximately 30 million stems in 1980 to 15.6 million stems in 1990 (55). Following implementation of an integrated disease management program, annual production losses were eventually reduced to 5% or less. However, due to the high cost of disease management, a few large farms now dominate the commercial markets.
| |

Fig. 8. Effects of bacterial blight in shadehouse. Photo by A. Alvarez. |
|
Once introduced into a new growing area, bacterial blight may result in 50 to 100% loss of plants. This devastating disease has limited anthurium production not only in Hawaii, but throughout the world where anthuriums are produced. By 1992, it had been reported in the Philippines, Guam, Australia, Florida, Jamaica, Puerto Rico, Martinique, Venezuela and Trindad and has since been reported in India (54) and the Netherlands (50). Bacterial blight affects most genera and species in the family Araceae (39).
Disease Symptoms
Early foliar symptoms start as water-soaked spots visible near the margins where hydathodes, filled with guttation fluid, serve as the most common port of entry (Figs. 9 and 10). Tissues surrounding the infected areas turn yellow. Water-soaked spots coalesce, eventually forming large necrotic zones at leaf margins (Fig. 11). The pathogen quickly moves into vascular tissues of petioles (Fig. 12) and stems, preventing the translocation of nutrients and water and producing symptoms of water stress (Fig. 13) that may resemble natural senescence (Fig. 14). The main stem of systemically infected plants turns dark brown (Fig. 15), and the growing point deteriorates (Fig. 16), eventually leading to death of the plant. When the spathe is infected the disease is often called "flower blight" (Fig. 17). Less frequently, bacteria enter stomates, forming circular water-soaked lesions surrounded by chlorotic zones (Fig. 18). Stomatal invasion often results in limited colonization of mesophyll tissues and does not necessarily lead to systemic infection.
| |
 |
|
 |
|
| |
Fig. 9. Guttation droplets at vein endings on leaf margins. Photo by A. R. Kuehnle. |
|
Fig. 10. Water-soaked lesions developing at leaf margins. Photo by W. Nishijima. |
|
 |
|
 |
|
Fig. 11. Typical blight symptoms showing necrotic zones surrounded by chlorotic tissues. Photo by W. Nishijima. |
|
Fig. 12. Discoloration of vascular elements in an infected invaded petiole. Photo by W. Nishijima. |
| |
 |
|
 |
|
| |
Fig. 13. Interveinal chlorosis on leaves characteristic of a systemic infection. Photo by W. Nishijima. |
|
Fig. 14. Systemic infection resulting in death of potted plants. Photo by T. Vowell. |
|
| |
 |
|
 |
|
| |
Fig. 15. Blackening and rotting of main stem. Photo by W. Nishijima. |
|
Fig. 16. Loss of leaves and decay at the growing point. Systemic infection Photo by W. Nishijima. |
|
| |
 |
|
 |
|
| |
Fig. 17. Blacking of the spathe in the ‘flower blight’ stage. Photo by T. Vowell. |
|
Fig. 18. Water-soaked lesions surrounding stomates on underside of leaf. Photo by A. Alvarez. |
|
Pathogen Detection and Monitoring
In early studies of bacterial blight, two physiologically distinct populations were found throughout Hawaiian farms. One produced large, clear zones of starch hydrolysis on differential media, and a second produced weak or no zones, indicating minimal capacity to hydrolyze starch. The starch negative strains often were associated with separate origins of planting stock (39). Subsequently, monoclonal antibodies were generated to characterize strains of X. axonopodis pv. dieffenbachiae associated with blight from outbreaks in separate shadehouses on several farms. The strains were serotyped (assigned to serogroups) based on their reactions to a panel of monoclonal antibodies. A comparison of 323 strains resulted in a clear separation of anthurium strains from those isolated from the other aroids (Fig. 19) (39). Fingerprint analysis using rep-PCR has revealed similar grouping of anthurium strains and a separation of strains from other aroids (38). An immunocapture PCR that shows promise for molecular diagnosis was recently developed using a Xanthomonas-specific monoclonal antibody in combination with specific primers in a multiplex PCR (32).
| |
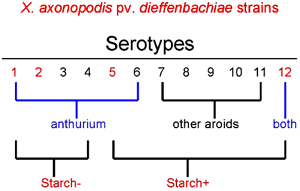
Fig. 19. Relationship between serotypes and capacity to hydrolyze starch among strains of X. axonopodis pv. dieffenbachiae. Strains from anthurium were in serotypes 1-6; strains from other aroids were mostly in serotypes 7-11. Nonpathogenic strains were often serotype 12, reacting only with a Xanthomonas-specific monoclonal antibody. |
|
Serotyping was used to trace the movement of the bacteria in shadehouses (2,4,45,47). Through culturing and immunodiagnostic tests it was found that the pathogen was spread by a number of methods including contaminated cutting tools, infected plant material, wind-driven rain or irrigation water, and aerosols (7,56). The pathogen does not survive long in soil and rarely infects through roots.
The capacity of X. axonopodis pv. dieffenbachiae to spread through aerosols was demonstrated using Andersen samplers and settling plates (7). Xanthomonads were recovered from air sampled over the plant canopy of three farms on rainy and cloudy days and settled on culture plates beneath the canopy. In this study, X. axonopodis pv. dieffenbachiae was captured from aerosols in relatively low numbers, primarily during rain and sprinkler irrigation events. Other studies (Venette, unpublished data) indicated that X. axonopodis pv. dieffenbachiae is not sensitive to aerosolization and could survive long enough to contaminate other plants within a shadehouse. X. axonopodis pv. dieffenbachiae was detected by samplers placed in the roadway 5 m (16.4 ft) downwind of a sprinkler irrigated block of anthuriums and behind a shadecloth barrier installed to preclude bacterial movement by splash droplets. This indicated that control procedures based on exclusion of the pathogen through a clean planting stock program may be thwarted unless appropriate precautions are taken. The risk is high if propagation areas for tissue-cultured plantlets are downwind of flower production areas. Tissue-cultured plantlets were often established on misting benches, and young wet tissues are highly susceptible to infection.
When 77 strains recovered from Andersen samplers were serotyped and tested for pathogenicity, 93% of the pathogenic strains were serotype 5, whereas 84% of the nonpathogenic strains were serotype 12. This study also revealed a direct correlation between pathogenicity and reactivity with a new monoclonal antibody, MAb Xcd108. Noteably, the new MAb reacted with most (95%) of the pathogenic strains recovered, whereas it reacted with only 8% of the nonpathogenic strains (7).
Latent Infections
The value of serotyping the strains became more obvious when cuttings destined for use as planting stocks were indexed for the presence of the pathogen. It was initially thought that symptomless cutting materials were pathogen free, so propagative materials grown at high elevation under cooler conditions were used as planting stocks for lower elevation farms. However, a study involving repeated assays of 1000 symptomless cuttings revealed that 0.4% were initially infected when placed into pots on greenhouse benches. Under overhead irrigation, the pathogen spread to neighboring plants. On the second assay five months later, X. axonopodis pv. dieffenbachiae was detected in 3.9% of the symptomless plants. Of 967 symptomless cuttings transplanted into the production field, 6.9% developed blight symptoms within the first year. During the three years of production, 20% of the plants exhibited blight symptoms, causing the planting to be abandoned (48). The risks associated with latent infections in symptomless cuttings convinced growers to develop a pathogen-free tissue-culture program, which remains the most important component of a successful disease management program for anthurium blight today.
Latent infection is the most difficult aspect of the disease to monitor, so a bioluminescent strain of X. axonopodis pv. dieffenbachiae was generated to visualize colonization of symptomless anthurium plants (5). The bioluminescent strain was also used to study the infection process, cultivar susceptibility to the pathogen, temperature affects on leaf colonization and response to biological control (16,17,18,19,20).
Cultural and Chemical Controls
Components of an integrated management program for anthurium blight include sanitation, disinfection of harvesting implements and containers, chemical sprays, modification of cultural practices, production of pathogen-free planting stocks in vitro, use of resistant cultivars, and biological control. Field sanitation, which involves removal of leaves showing early infections and elimination of systemically infected plants, was the principal method of reducing anthurium blight in early years (42). Disinfection of cutting tools is important to prevent the spread of blight, since plant materials which show no symptoms have the potential for latent infection and the pathogen is spread during harvest. These two approaches, while useful, are insufficient for stopping disease spread.
Some chemicals and antibiotics were used with limited success at early stages of the blight outbreak in Hawaii (3,6,44), but these methods of control were later abandoned. Cultural practices, with a focus on nitrogen fertilization, was then examined (40). Chase (9) suggested that lower fertilizer rates for potted anthuriums could result in reduced disease and greater flower production. Sakai (51) reported that higher levels of ammonium fertilizer led to higher amounts of amino compounds in guttation fluid when compared to nitrate fertilizers. He suggested that this promoted bacterial multiplication in the guttation fluid. The use of nitrate or inorganic fertilizers for plant growth was expected to reduce the amount of amino compounds in guttation fluid and thereby reduce blight incidence (52,53). Although greenhouse trials indicated a relationship between fertilizer treatments and blight susceptibility, field trials were inconclusive (22,24).
Growing plants under plastic or glass houses coupled with drip irrigation rather than overhead or sprinkler irrigation reduced the spread of the bacteria through aerosols and water splash and significantly reduced the incidence of blight in anthurium seedling culture (29). Growing anthuriums under cool and shaded conditions slows the progression of the disease. Inoculated plants exposed to temperatures greater than 31°C (87.8°F) were more susceptible to disease than inoculated plants exposed to 26°C (78.8°F) or lower temperatures (Fig. 20) (8). This difference in temperature can be regulated in commercial shadehouses by strategically increasing airflow.
| |

Fig. 20. Effect of temperature on anthurium blight development. Plants grown at 26°C developed few symptoms following inoculation while plants grown at 31°C and higher show severe blight and heat stress. Photo by A. Alvarez. |
|
Tissue Cultured Anthuriums
The best single means for blight disease management entails the use of plant material which is guaranteed to be pathogen free. Tissue cultured plants, although highly regarded and recommended to growers, have the potential of latent infection with X. axonopodis pv. dieffenbachiae (46,49). Microplants inoculated with a dilution series of the pathogen were killed when the pathogen was incorporated into the liquid medium at high populations, but microplants inoculated at very low population levels remained symptomless for 10 months. Furthermore, the pathogen was reisolated from the symptomless plants up to the time of deflasking a year later. Addition of coconut water to stimulate growth of microplants provided a carbon source for quiescent bacteria, which then formed cloudy suspensions in vitro (46).
A triple indexing protocol developed for anthuriums involves several steps, as follows: (i) axillary buds are placed into culture to initiate the first generation of plantlets; (ii) the bases of the plantlets are sectioned and tested for the presence of microorganisms using nutrient broth; (iii) if the test is negative, the plantlets are cut into three nodal sections; (iv) each nodal section is placed into liquid half-strength Murashige and Skoog medium with 15% coconut water; (v) this procedure is repeated two additional times if the plantlets test negative in nutrient broth. If the material tests positive at any point, the previously cultured material will be eliminated from further propagation (56). Triple indexing ensures that tissue-cultured plants do not serve as sources of inoculum.
Development of Resistant Cultivars
Many of the early cultivars developed for Hawaii’s anthurium industry before 1980 were bred mainly for resistance to anthracnose, while incorporating other desirable horticultural traits such as color, shape, and yield (28). Most of the cultivars were susceptible to blight, but in varying degrees. Resistant cultivars of A. antioquiense may become infected with the pathogen, but rarely develop systemic infection. The introduction of A. antioquense in crosses with A. andraeanum have resulted in tolerant offspring (29,31,33,36). Anthurium andraeanum and A. antioquiense cultivars also showed differential susceptibility to bacterial blight in foliar and systemic infection phases (16).
Although some anthuriums are tolerant to X. axonopodis pv. dieffenbachiae, natural genetic resistance to bacterial blight is not present in anthuriums, and breeding plants for tolerance through traditional means is time consuming (30). Genetic engineering serves as a means of introducing resistance genes from non-plant origins into anthurium plants. Agrobacterium-mediated gene transfer has been used to successfully transform anthuriums (34). Genes that code the antibacterial peptides attacin and cecropin have been isolated from the cecropia moth (Hyalophora cecropia) and genetically engineered into anthuriums (33,35,36). Transgenic anthurium plants expressing attacin were less susceptible to X. axonopodis pv. dieffenbachiae and had fewer numbers of bacteria present when compared to non-transgenic plants (30).
Kuehnle et. al (35) reported that two cultivars transformed to express the Shiva-1 lytic peptide (a synthetic analog of cecropin B) significantly resisted anthurium blight. Comparisons were made between transgenic and non-transgenic lines of each cultivar as well as to a susceptible ‘Rudolph’ and tolerant ‘Kalapana’ lines used as controls. Resistance to infection was evaluated using a bioluminescent strain of X. axonopodis pv. dieffenbachiae to determine the amount of tissue colonized in wild-type and transgenic plants. Extensive bacterial colonization of major veins leading to the petiole was visualized by their bioluminescence in symptomless leaves (Figs. 21A and 21B). Other plants showed small sites of infection at leaf margins whereas bioluminescence revealed a far greater level of tissue colonization (Figs. 22A and 22B). Disease progression in a transgenic line of ‘Paradise Pink’ was significantly reduced compared to the non-transgenic control, indicating increased tolerance. In contrast, one transgenic line of ‘Tropic Flame’ had increased susceptibility to blight, possibly due to the reduced transcription of the transgene. Other lines of ‘Tropic Flame’ did not differ significantly from the controls. Additional testing of lines under field conditions is necessary before resistant transgenic cultivars are released.
| |
 |
|
 |
|
| |
Fig. 21A-21B. Evaluation of transgenic anthurium for blight resistance using a bioluminescent strain of X. axonopodis pv. dieffenbachiae. No signs of infection are visible on the leaf (A). The leaf perimeter has been traced onto the x-ray film (B), where blackened areas reveal that bacteria have colonized the main veins leading to the petiole. Photos by T. Fujii. |
|
| |
Fig. 22A-22 B. An early stage of infection showing symptoms at the right leaf margin (A). The x-ray image (B) shows that bacteria have already colonized veins leading to the petiole. The film also reveals two additional infection sites at the leaf margins (A). Photos by T. Fujii. |
|
Biological Control of Anthurium Blight
The use of beneficial organisms, natural or modified, to control the effects of undesirable organisms was proposed to anthurium growers during the initial stages of the bacterial blight epidemic (10). Cultures of microorganisms were soon isolated from internal petiole tissues of anthuriums and examined as a means for biological control of bacterial blight (12). However, foliar applications of microorganisms antagonistic to X. axonopodis pv. dieffenbachiae resulted in inconsistent or insignificant control of the disease (13,14).
In later studies, Fukui et al. (17) isolated bacteria from the guttation fluids of susceptible anthurium cultivars (‘Marian Seefurth’ and UH1060) that did not succumb to infection by X. axonopodis pv. dieffenbachiae even under high inoculum pressure. Individually these beneficial bacteria were not effective in suppressing multiplication of X. axonopodis pv. dieffenbachiae in guttation fluids, but were effective when applied in combination (17,18). Foliar applications of the bacterial community reduced infection of anthurium leaves through hydathodes and wounds (18). Using a combination of bacteriological tests, fatty acid analysis, and 16S rDNA sequence analysis, the strains were identified as Sphingomonas chlorophenolica, Microbacterium testaceum, Brevundimonas vesicularis, and Herbaspirillum rubrisulbalbicans. All four species survived on the surfaces of microplants up to two months and were effective in protecting microplants against infection (1). Striking differences were observed when susceptible cultivar ‘Rudolph’ was inoculated with X. axonopodis pv. dieffenbachiae and either treated (sprayed weekly) or not treated with the biocontrol agents. Non-treated plants developed typical blight symptoms after inoculation with the pathogen and died. In contrast, 75% of the treated plants survived (Fig. 23) and produced flowers after 22 weeks (Fig. 24).
 |
|
 |
|
Fig. 23. Disease reduction on ‘Marian Seefurth’ plants treated with beneficial bacteria (four plants on right) compared to four non-treated plants (left) 28 days after inoculation with X. axonopodis pv. dieffenbachiae. In repeated trials disease was reduced by 25 to 40% on treated plants, whereas all non-treated plants died within 28 to 35 days after inoculation. Photo by A. Alvarez. |
|
Fig. 24. ‘Marian Seefurth’ plants after continued weekly post-inoculation treatments. Diseased plants (Fig. 23) eventually recovered and produced flowers. Photo by A. Alvarez. |
Fujii et al (15) demonstrated that biological control could be used simultaneously with genetic modification of anthurium cultivars. The cultivars ‘Paradise Pink’ and ‘Mauna Kea’ were engineered to express the Shiva-1 lytic peptide, an antibacterial peptide, but did not inhibit the four species of beneficial bacteria when applied to these cultivars (35,37).
Growth Stimulation of Microplants Using Beneficial Bacteria
Biostimulation was observed as an unexpected outcome in studies involving anthurium treatment for biological control (1). Stage 4 tissue cultured microplants treated with the bacterial consortium discussed above had stronger root systems and greater survival rates during transplant to individual pots than non-treated plants. Treated plants were more vigorous, flowered sooner, and were larger in plant height, leaf area, leaf number, and shoot and root dry weights. Growth effects were most striking on A. andraeanum cultivars ‘Rudolph’, ‘Marian Seefurth’ and several numbered cultivars.
Summary and Future Perspectives
Growers have been struggling with anthurium blight since the onset of the major disease outbreaks in the early 1980s. Good cultural practices help to reduce losses when growing anthuriums, but they are insufficient for blight control. Use of symptomless cuttings for propagation is a risk even when propagative materials are grown at high elevation, so establishment of pathogen-free microplants in vitro is essential for large-scale anthurium production. The A. andraeanum cultivars, which are the mainstay of Hawaiian anthurium production because of the large showy flowers used in floral arrangements, are susceptible to blight. While the smaller, upright A. antioquiense cultivars with blight tolerance are now incorporated into the breeding programs, hybrid cultivars lack the desirable heart-shaped spathe. Thus, molecular methods are now being used for transferring resistance genes into susceptible A. andraeanum cultivars. Biological control is also a long-term approach. Beneficial bacteria have been isolated, characterized, and evaluated for both biocontrol and biostimulation in greenhouse and larger shadehouse experiments. They show promise, but protection is not complete and they have not yet become incorporated into mainstream anthurium production practices. Currently, combinations of tissue culture, sanitation, cultural practices, and breeding for plant resistance are used for disease management, while transgenic plants and biocontrol are being developed and tested.
Literature Cited
1. Alvarez, A., and Mizumoto, C. 2001. Bioprotection and stimulation of aroids with phylloplane bacteria. Phytopathology. 91:S3.
2. Alvarez, A., and Norman, D. 1993. Alternatives for control of anthurium blight using information gained from epidemiological studies. Pages 17-21 in: Proc. Hawaii Anthurium Ind. Conf. 6th. K. M. Delate and E. R. Yoshimura, eds. Hawaii Inst. Trop. Agric. Human Res., University of Hawaii, Honolulu.
3. Alvarez, A., Lipp, R., and Bushe, B. 1989. Resistance of bacteria to antibiotics used for control of anthurium blight. Pages 11-12 in: Proc Anthurium Blight Conf., 2nd. J. A. Fernandez and W. T. Nishijima, eds. Hawaii Inst. Trop. Agric. Human Res., University of Hawaii, Honolulu.
4. Alvarez, A., Lipp, R., and Norman, D. 1988. Detection and serological studies. Pages 11-15 in: Proc. Anthurium Blight Conf., 1st. A. M. Alvarez, ed. Hawaii Inst. Trop. Agric. Human Res., University of Hawaii, Honolulu.
5. Alvarez, A., McElhaney, R., and Fukui, R. 1993. Studies of the infection process in anthurium blight using a bioluminescent strain of Xanthomonas campestris pv. dieffenbachiae. Pages 31-37 in: Proc. Hawaii Anthurium Ind. Conf. 6th. K. M. Delate and E. R. Yoshimura, eds. Hawaii Inst. Trop. Agric. Human Res., University of Hawaii, Honolulu.
6. Alvarez, A., Norman, D., and Lipp, R. 1991. Epidemiology and control of anthurium blight. Pages 12-18 in: Proc. Anthurium Blight Conf., 4th. A. M. Alvarez, D. C. Deardorff, and K. B. Wadsworth, eds. Hawaii Inst. Trop. Agric. Human Res., University of Hawaii, Honolulu.
7. Alvarez, A., Venette, J., and Norman, D. 1992. Relationship of aerosols to anthurium blight. Pages 20-26 in: Proc. Anthurium Blight Conf., 5th. K. M. Delate and C. H. M. Tome, eds. Hawaii Inst. Trop. Agric. Human Res., University of Hawaii, Honolulu.
8. Alvarez, A., Lipp, R., Norman, D., and Gladstone, L. 1990. Epidemiology and control of anthurium blight. Pages 27-30 in: Proc. Anthurium Blight Conf., 3rd. A. M. Alvarez, ed. Hawaii Inst. Trop. Agric. Human Res., University of Hawaii, Honolulu.
9. Chase, A. R. 1988. Chemical and nutritional aspects of controlling Xanthomonas diseases on Florida ornamentals. Pages 32-34 in: Proc. Anthurium Blight Conf., 1st. A. M. Alvarez, ed. Hawaii Inst. Trop. Agric. Human Res., University of Hawaii, Honolulu.
10. Cook, J. R. 1988. Biological control: some concepts, and the potential for application to bacterial blight of anthurium. Pages 35-36 in: Proc. Anthurium Blight Conf., 1st. A. M. Alvarez, ed. Hawaii Inst. Trop. Agric. Human Res., University of Hawaii, Honolulu.
11. Deardoff, D. C. 1991. Plant bacterial diseases and their control. Pages 66-69 in: Proc. Anthurium Blight Conf., 4th. A. M. Alvarez, D. C. Deardorff, and K. B. Wadsworth, eds. Hawaii Inst. Trop. Agric. Human Res., University of Hawaii, Honolulu.
12. Fernandez, J. A., Tanabe, M. J., Moriyasu, P., and Duffy, B. 1989. Biological control. Pages 27-29 in: Proc. Anthurium Blight Conf., 2nd. J. A. Fernandez, W. T. Nishijima, eds. Hawaii Inst. Trop. Agric. Human Res., University of Hawaii, Honolulu.
13. Fernandez, J. A., Tanabe, M. J., Moriyasu, P., and Wolff, W. J. 1990. Biological control. Pages 41-43 in: Proc. Anthurium Blight Conf., 3rd. A. M. Alvarez, ed. Hawaii Inst. Trop. Agric. Human Res., University of Hawaii, Honolulu.
14. Fernandez, J. A., Tanabe, M. J., Wolff, W. J., and Moriyasu, P. 1991. Biological control. Pages 28-30 in: Proc. Anthurium Blight Conf., 4th. A. M. Alvarez, D. C. Deardorff, and K. B. Wadsworth, eds. Hawaii Inst. Trop. Agric. Human Res., University of Hawaii, Honolulu.
15. Fujii, T. M., Alvarez, A., Fukui, R., Obsuwan, K., and Kuehnle, A. R. 2002. Effect of transgenic anthuriums producing the Shiva-1 lytic peptide on beneficial bacteria. Phytopathology. 92:S27
16. Fukui, H., Alvarez, A. M., and Fukui, R. 1998. Differential susceptibility of anthurium cultivars to bacterial blight in foliar and systemic infection phases. Plant Dis. 82:800-806.
17. Fukui, R., Fukui, H., and Alvarez, A. M. 1999. Comparisons of single versus multiple bacterial species on biological control of anthurium blight. Phytopathology. 89:366-373.
18. Fukui, R., Fukui, H., and Alvarez, A. M. 1999. Suppression of bacterial blight by a community isolated from the guttation fluids of anthuriums. Appl. Environ. Microbiol. 65:1020-1028.
19. Fukui, R., Fukui, H., and Alvarez, A. M. 1999. Effect of temperature on the incubation period and leaf colonization in bacterial blight of anthurium. Phytopathology. 89:1007-1014.
20. Fukui, R., McElhaney, R., Nelson, S. C., and Alvarez, A. M. 1996. Relationship between symptom development and actual sites of infection in leaves of anthurium inoculated with a bioluminescent strain of Xanthomonas campestris pv. dieffenbachiae. Appl. Environ. Microbiol. 62:1021-1027.
21. Hayward, A. C. 1972. A bacterial disease of anthurium in Hawaii. Plant Dis. Rep. 56:904-908.
22. Higaki, T., Imamura, J. S., and Moniz, D. 1992. Nutritional and cultural effects on bacterial blight of anthurium. Pages 44-45. Proc. Anthurium Blight Conf., 5th. K. M. Delate, and C. H. M. Tome, eds. Hawaii Inst. Trop. Agric. Human Res., University of Hawaii, Honolulu.
23. Higaki, T., Watson, D. P., and Leonhardt, K. W. 1983. Anthurium culture in Hawaii. Cooperative Extension Service Circular 420, College of Tropical Agriculture and Human Resources, University of Hawaii, Manoa.
24. Higaki, T., Imamura J., Tanabe, M., Nishijima, W., Hara, A., Deardoff, D., and Sewake, K. 1990. Nutritional and cultural effects on anthurium bacterial blight. Pages 7-11 in: Proc. Anthurium Blight Conf., 3rd. A. M. Alvarez, ed. Hawaii Inst. Trop. Agric. Human Res., University of Hawaii, Honolulu.
25. Hiloweb. 2003. Brief history of the anthurium tropical flower industry on the Big Island of Hawaii. Online. Hiloweb.
26. Kamemoto, H. 1981. Anthurium breeding in Hawaii. Aroideana. 4:77-86.
27. Kamemoto, H. 1988a. History and development of anthuriums in Hawaii. Pages 4-5 in: Proc. Anthurium Blight Conf., 1st. A. M. Alvarez, ed. Hawaii Inst. Trop. Agric. Human Res., University of Hawaii, Honolulu.
28. Kamemoto, H. 1988b. Breeding for resistance to bacterial blight of anthuriums. Pages 17-18 in: Proc. Anthurium Blight Conf., 1st. A. M. Alvarez, ed. Hawaii Inst. Trop. Agric. Human Res., University of Hawaii, Honolulu.
29. Kamemoto, H., and Kuehnle, A. 1989. Breeding for blight resistance: a progress report. P. 10 in: Proc. Anthurium Blight Conf., 2nd. J. A. Fernandez and W. T. Nishijima, eds. Hawaii Inst. Trop. Agric. Human Res., University of Hawaii, Honolulu.
30. Kamemoto, H., and Kuehnle, A. 1996. Breeding Anthuriums in Hawaii. University of Hawaii Press, Honolulu.
31. Kamemoto, H., Kuehnle, A., Kunisaki, J., Aragaki, M., Higaki, T., and Imamura, J. 1990. Breeding for bacterial blight resistance in anthurium. Pages 45-48 in: Proc. Anthurium Blight Conf., 3rd. A. M. Alvarez, ed. Hawaii Inst. Trop. Agric. Human Res., University of Hawaii, Honolulu.
32. Khoodoo, M. H. R., Sahin, F., and Jaufeerally-Fakim, Y. 2005. Sensitive detection of Xanthomonas axonopodis pv. dieffenbachiae on Anthurium andraeanum by immunocapture-PCR (IC-PCR) using primer designed from sequence characterized amplified regions (SCAR) of the blight pathogen. Eur. J. Plant Pathol. 112:379-390.
33. Kuehnle, A. R., Chen, F. C., and Jaynes, J. M. 1993. Status of genetically engineered anthuriums. Pages 7-8 in: Proc. Hawaii Anthurium Ind. Conf. 6th. K. M. Delate and E. R. Yoshimura, eds. Hawaii Inst. Trop. Agric. Human Res., University of Hawaii, Honolulu.
34. Kuehnle, A. R., Chen, F. C., Sugii, N., and Jaynes, J. M. 1991. Engineering of blight resistance in Anthurium. Pages 42-43 in: Proc. Anthurium Blight Conf., 4th. A. M. Alvarez, D. C. Deardorff, and K. B. Wadsworth, eds. Hawaii Inst. Trop. Agric. Human Res., University of Hawaii, Honolulu.
35. Kuehnle, A. R., Fujii, T., Mudalige, R., and Alvarez, A. 2004. Gene and genome mélange in breeding of Anthurium and Dendrobium Orchid. Acta Hort. (ISHS) 651:115-122.
36. Kuehnle, A. R., Chen, F. C., Jaynes, J. M., Norman, D., and Alvarez, A. 1992. Engineering blight resistant Anthurium: a progress report. Pages 17-18. Proc. Anthurium Blight Conf., 5th. K. M. Delate, and C. H. M. Tome, eds. Hawaii Inst. Trop. Agric. Human Res., University of Hawaii, Honolulu.
37. Kuehnle, A. R., Fujii, R., Chen, F. C., Alvarez, A., Sugii, N., Fukui, R., and Aragon, S. L. 2004b. Peptide biocides for engineering bacterial blight tolerance and susceptibility in cut flower anthurium. HortScience 39:1327-1331.
38. Louws F. J., and Alvarez, A. 2000. Genetic diversity of xanthomonads isolated
from aroids determined by rep-PCR. Phytopathology. 90:S47.
39. Lipp, R. L., Alvarez, A. M., Benedict, A. A., and Berestecky, J. 1992. Use of monoclonal and pathogenicity tests to characterize strains of Xanthomonas campestris pv. dieffenbachiae from Aroids. Phytopathology. 82:677-682.
40. Mills, H. A. 1989. Cultural practices and anthurium nutrition. Pages 40-42 in: Proc. Anthurium Blight Conf., 2nd. J. A. Fernandez, and W. T. Nishijima, eds. Hawaii Inst. Trop. Agric. Human Res., University of Hawaii, Honolulu.
41. Neal, M. C. 1965. In Gardens of Hawaii. Bishop Museum Press, Honolulu.
42. Nishijima, W. T. 1988. Anthurim blight: an overview. Pages 6-8 in: Proc. Anthurium Blight Conf., 1st. A. M. Alvarez, ed. Hawaii Inst. Trop. Agric. Human Res., University of Hawaii, Honolulu.
43. Nishijima, W. T. 1994. Diseases. Pages 13-18 in: Anthurium culture in Hawaii. HITHAR Research Extension Series 152. T. Higaki, J. S. Lichty, and D. Moniz, eds. College of Tropical Agriculture and Human Resources, University of Hawaii, Honolulu.
44. Nishijima, W., and Chun, M. 1991. Chemical control of anthurium blight. Pages 21-23 in: Proc. Anthurium Blight Conf., 4th. A. M. Alvarez, D. C. Deardorff, and K. B. Wadsworth, eds. Hawaii Inst. Trop. Agric. Human Res., University of Hawaii, Honolulu.
45. Norman, D., and Alvarez, A. 1989. A rapid method for presumptive identification of Xanthomonas campestris pv. dieffenbachiae and other xanthomonads. Plant Dis. 73:654-658.
46. Norman, D. J., and Alvarez, A. M. 1994. Latent infections of in vitro anthurium caused by Xanthomonas campestris pv. dieffenbachiae. Plant Cell Tissue Organ Cult. 39:55-61.
47. Norman, D. J., and Alvarez, A. M. 1994. Rapid detection of Xanthomonas campestris pv. dieffenbachiae in anthurium plants with a miniplate enrichment / ELISA system. Plant Dis. 78:954-958.
48. Norman, D. J. and Alvarez, A. M. 1996. Monitoring the spread of Xanthomonas campestris pv. dieffenbachiae introduced from symptomless Anthurium cuttings into production fields. J. Amer. Soc. Hort. Sci. 121:582-585.
49. Norman, D., and Alvarez, A., Lipp, R. 1993. Latent infections of Xanthomonas campestris pv. dieffenbachiae in tissue-cultured anthurium. Pages 12-16 in: Proc. Hawaii Anthurium Ind. Conf. 6th. K. M. Delate and E. R. Yoshimura, eds. Hawaii Inst. Trop. Agric. Human Res., University of Hawaii, Honolulu.
50. OEPP/EPPO. 2004. Diagnostic protocols for regulated pests, Xanthomonas axonopodis pv. dieffenbachiae. OEPP/EPPO Bull. 34:183-186.
51. Sakai, D. S. 1990. The effect of nitrate and ammonium fertilizer on the contents of anthurium guttation fluid. Pages 18-19 in: Proc. Anthurium Blight Conf., 3rd. A. M. Alvarez, ed. Hawaii Inst. Trop. Agric. Human Res., University of Hawaii, Honolulu.
52. Sakai, D. S. 1991. The effect of nitrogen fertilizer levels on amino compounds in guttation fluid of anthurium and incidence of bacterial blight. Pages 51-52 in: Proc. Anthurium Blight Conf., 4th. A. M. Alvarez, D. C. Deardorff, and K. B. Wadsworth, eds. Hawaii Inst. Trop. Agric. Human Res., University of Hawaii, Honolulu.
53. Sakai, W. S., Okimura, S., Hanohano, T., Furutani, S. C., Sakai, D. S. 1992. A detailed study of nitrogen fertilization, glutamine production, and systemic blight on anthurium cultivars Ellison Onizuka and Calypso. Pages 47-50 in: Proc. Anthurium Blight Conf., 5th. K. M. Delate and C. H. M. Tome eds. Hawaii Inst. Trop. Agric. Human Res., University of Hawaii, Honolulu.
54. Sathyanarayana, N., Reddy, O. R., and R. L. Rajak. 1998. Interception of Xanthomonas campestris pv. dieffenbachiae on anthurium plants from the Netherlands. Plant Dis. 82:262.
55. Shehata, S. 1992. Supply-demand and market analysis of the cut-flower industry: a focus on the Hawaiian anthurium industry. Pages 35-38 in: Proc. Anthurium Blight Conf., 5th. K. M. Delate and C. H. M. Tome, eds. Hawaii Inst. Trop. Agric. Human Res., University of Hawaii, Honolulu.
56. Tanabe, M., Fernandez, J., Moriyasu, P., Crane, S., Wolff, W., and Liu, R-W. 1992. Anthurium in vitro triple indexing. Pages 8-11 in: Proc. Anthurium Blight Conf., 5th. K. M. Delate and C. H. M. Tome, eds. Hawaii Inst. Trop. Agric. Human Res., University of Hawaii, Honolulu.
57. USDA/HASS. 2005. Hawaii Agricultural Statistics. Online. Hawaii Agric. Statistics, Nat. Agric. Statistics Serv. (NASS), Honolulu.
58. Van Uffelen, A. 1996. Creative Flower Arranging with Anthurium. Kosmos-Z&K Uitgevers B.V., Utrecht.
