Heffer Link, V., M.L. Powelson, and K.B. Johnson. 2002. Oomycetes.
The Plant Health Instructor. DOI: 10.1094/PHI-I-2002-0225-01
Updated 2012
INTRODUCTION:
The Oomycetes, also known as water molds, are a large group of terrestrial and aquatic eukaryotic organisms. Although they superficially resemble fungi in mycelial growth and mode of nutrition, molecular studies and distinct morphological characteristics place them in the kingdom Chromalveolata (phylum Heterokontophyta, the 'stramenopiles') with brown and golden algae and diatoms.
The terrestrial Oomycetes are primarily parasites of vascular plants, and include several very important plant pathogens.
Aphanomyces, in the order Saprolegniales, causes a root rot of a wide range of hosts, including pea, snap bean, and sugar beet.
 |
Root rot of pea, caused by
Aphanomyces
euteiches. (Courtesy D. Hagedorn) |
The order Peronosporales contains three families of plant pathogens. In the family Peronosporaceae,
Plasmopara, Peronospora,
Pseudoperonospora,
Sclerospora, and
Bremia are obligate parasites that cause serious foliar diseases known as
downy mildews on many host plants such as grape, broccoli, onion, cucurbits, sorghum, and lettuce.
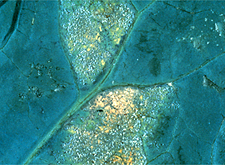
|
Broccoli leaf with signs of downy mildew, caused by
Peronospora
parasitica.(Courtesy G.A. Payne) |
The family Pythiaceae contains obligate and nonobligate parasites, and includes the important pathogen genera
Pythium and Phytophthora.
Pythium species cause a variety of diseases including root rots of numerous plant species, Pythium blight of turf, and
damping-off, which involves seed rot and pre- and post-emergence seedling death.
Phytophthora species cause late blight of potato and tomato, foliar blights on peppers and cucurbits, and root or stem rots of many plant species.
Phytophthora ramorum is a newly identified foliar pathogen that causes a disease called sudden oak death (also known as ramorum blight) on many trees and shrubs, and is a serious threat to forests and nurseries.
Lessons on several diseases caused by oomycetes are available.
 |
 |
|
Damping-off of bedding plant seedlings due to
Pythium. (Courtesy M.Daughtrey, copyright-free) |
Conifer seedlings on the left are stunted and yellowed due to Phytophthora root rot.(Courtesy P.B. Hamm) |

|

|
|
Symptoms and signs of late blight, caused by
Phytophthora infestans, on a potato leaf. (Courtesy M. Powelson) |
Advanced symptoms of sudden oak death in tanoak caused by
Phytophthora ramorum (Courtesy E. Hansen) |
The family Albuginaceae includes
Albugo, an obligate parasite that produces a generally mild disease called
white rust (not a true rust) on the stems, leaves, and fruit of cruciferous plants such as radish, horseradish, and several weed species.
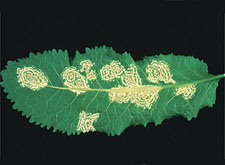
|
White rust on leaf of cruciferous plant, caused by
Albugo candida. (Courtesy P.A. Williams, copyright-free) |
Although Oomycetes have previously been referred to as "lower fungi," they differ from fungi in several features. Oomycete cell walls contain cellulose, beta glucans, and the amino acid hydroxyproline, but do not contain chitin, which occurs in the cell walls of true fungi. The vegetative state of Oomycetes is diploid, whereas true fungi are haploid or dikaryotic. Oomycetes produce hyphae that are
nonseptate (coenocytic), i.e. lacking in cross walls.
Asexual reproduction occurs in most Oomycetes by the formation of a structure called a
sporangium (pl. sporangia) that arises on a specialized hypha termed a
sporangiophore. Sporangia differ among various Oomycetes pathogens with respect to the shape of the sporangium, its mode of germination, the location of sporangia with respect to the host tissue, and the structure of the sporangiophores. In
Aphanomyces, sporangia are filamentous and resemble vegetative hyphae. In
Peronospora and other genera causing downy mildews, sporangiophores are branched and tree-like, with a single sporangium at the tip of each branch, and are often found emerging from the stomates.
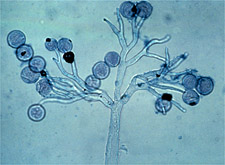
|
Sporangia and sporangiophore of
Peronospora parasitica, causal agent of downy mildew of cabbage. (Courtesy G.A. Payne)
|
Sporangial shape in
Pythium ranges from filamentous in some species to round in other species. Sporangia of
Phytophthora species are often lemon-shaped.
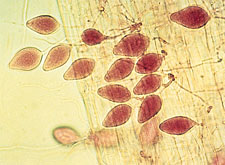
|
Lemon-shaped sporangia of
Phytophthora megasperma. (Courtesy F.A. Gray)
|
In
Albugo, an unbranched sporangiophore produces a chain of sporangia. Clusters of sporangia beneath the plant epidermis form blisters that rupture as sporangia are released.
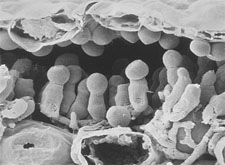
|
Sporangia of
Albugo
candida are produced on short sporangiophores beneath the epidermis of a mustard leaf. (Courtesy M.F. Brown and H.G. Brotzman) |
Two mechanisms for germination of sporangia are known. The type of mechanism is often species-specific; however, some species can germinate by either mechanism depending on temperature or moisture conditions. In many of the foliar pathogens (e.g.
Peronospora), sporangia germinate
directly on the surface of a susceptible plant by the formation of a
germ tube. A sporangium that germinates in this way is often referred to as a spore or conidium. Wind dispersion of sporangia of foliar pathogens is common.
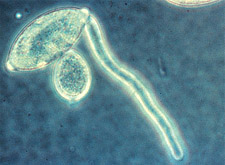
|
Direct germination of a sporangium by germ tube in
Phytophthora
infestans. (Courtesy H.D. Thurston)
|
In most of the soil- and water-inhabiting genera, sporangia germinate
indirectly by producing
zoospores, which are asexual spores that are motile by means of two dissimilar flagella. The presence of a fibrous, anteriorly directed "tinsel" flagellum with hairs and a posteriorly directed "whiplash" flagellum also differentiates oomycetes from fungi, and supports their classification in the Kingdom Stramenopila.
In
Aphanomyces and species of
Phytophthora, differentiation of zoospores occurs in the sporangium. In contrast, in many
Pythium species, the sporangium produces a short tube to which is connected a secondary structure referred to as a
vesicle. Zoospores are partially differentiated in the sporangium, and transferred through the tube into the vesicle for final development and release.
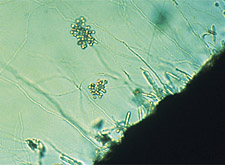
|
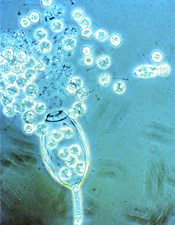
Zoospores being released from a sporangium of
Phytophthora. (Used by permission of F.W. Schwenk, Kansas State University)
|
|
Zoospores are cleaved in sporangia borne at terminal ends of hyphae of
Aphanomyces
euteiches. (Courtesy M.F. Heimann, copyright-free) |
|
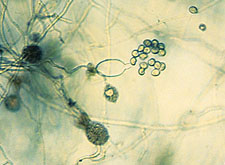
|
Pythium
undulatum, with round sporangia and thin vesicle surrounding zoospores just prior to release. (Courtesy P.B. Hamm) |
Sporangia of some species are capable of germinating either by direct germ tube formation or indirectly by zoospore formation. The type of germination is influenced most strongly by temperature; for example, in
Phytophthora infestans (the cause of late blight), cooler temperatures favor the formation of zoospores. For some soil-inhabiting species (e.g.
Phytophthora cryptogea), the type of sporangial germination is also influenced by soil moisture; in general, very wet or saturated soil conditions induce zoospore formation, whereas slightly drier conditions favor direct germination.
Dispersion of zoospores occurs as they swim in water through soil pores or on the soil surface. They are often attracted by sugars and amino acids exuded by plant roots. Zoospores also can be dispersed passively in flowing water over longer distances, contaminating streams, canals, and ponds used for irrigation water. On susceptible plant surfaces, zoospores encyst and then germinate by producing a germ tube.
Sexual reproduction in Oomycetes occurs between two dissimilar gametangia: a large round
oogonium containing one to several eggs, and a smaller
antheridium that fertilizes the oogonium. If the antheridium is located at the side of the oogonium, the arrangement is termed
paragynous. In an
amphigynous arrangement, the oogonium grows through the antheridium, which remains as a collar at its base.
|
|
|
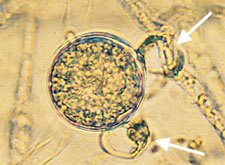
Paragynous arrangement of oogonium and antheridia in
Pythium. (Courtesy P.B. Hamm) (arrows indicate antheridia) |
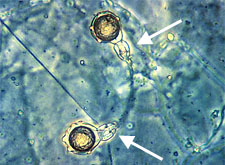
Amphigynous arrangement of oogonium and antheridium in
Phytophthora
cambivora. (Courtesy P.B. Hamm) (arrows indicate antheridia) |
In
homothallic species, fertilization can occur in a single strain. In
heterothallic species, two strains of opposite mating types are required for fertilization. In both homothallic and heterothallic species, fertilization results in a thick-walled zygote called an
oospore. Oospores function as resting spores. They are produced in infected plant tissue, and are released into soil as the plant tissue degrades. They germinate either directly by a germ tube with or without a sporangium on the end, or indirectly by the formation of a vesicle with zoospores. As with sporangia, the type of germination is species and environment dependent.
|
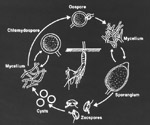
|
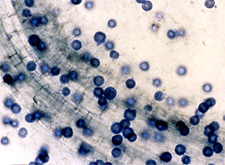
Mature oospores of
Aphanomyces within
an infected root. (Courtesy Jennifer Parke) |
|
Disease cycle of
Phytophthora cactorum. (Courtesy Jennifer Parke) Click image for an enlarged view. |
MATERIALS
- Fresh specimens of downy mildew, late blight or other foliar blights, damping-off, and white rust, labeled with disease name and pathogen genus
- Grass leaves inoculated with
Pythium spp.
- Dissecting microscopes
- Compound microscopes
- Razor blades
- Lactophenol cotton blue stain
- Clear Scotch tape (single- or double-sided)
- Microscope slides
- Coverslips
- Cultures and/or baits of Oomycetes showing sexual and asexual structures
- Prepared microscope slides (Plasmopara,
Peronospora,
Albugo)
- Pea or alfalfa seedlings
- Aqueous suspension of zoospores of
Aphanomyces or
Pythium
CLICK HERE FOR INSTRUCTOR'S NOTES
PROCEDURES
A. Examination of fresh diseased material for
symptoms of disease.
Examine disease specimens macroscopically. Record the disease name and pathogen genus on the following chart. Describe the symptoms you observe. Which physiological function(s) of the plant are most likely impaired by the disease?
B. Observation of fresh diseased material for
signs of disease.
1. When foliar (disease) symptoms of late blight or downy mildew are present, examine diseased leaf tissue with a dissecting microscope to locate areas of sporulation (production of sporangia). Use the
Scotch tape technique to make a spore mount. Touch the sticky side of a piece of clear Scotch tape to the sporulating area. Apply the tape, sticky-side down, to a drop of water on a microscope slide. Single-sided tape functions as its own coverslip; if double-stick tape is used, place a coverslip on top of it. Examine first under low, then under high power using a compound microscope. Observe the shape of the sporangia and their arrangement on the sporangiophore. Note the characteristic branching patterns of sporangiophores associated with different genera. Note also the nonseptate (coenocytic) hyphae typical of Oomycetes.
2. Use the
lactophenol
cotton blue stain to observe
Pythium-infected grass leaves for oospores. With a razor blade slice thin sections of plant tissue from the advancing margin of a lesion. Place the tissue in a drop of lactophenol cotton blue on a microscope slide. Apply a coverslip, and examine first under low, then under high power using a compound microscope. Look for round, thick-walled oospores within the cells of the leaf tissue.
3. Record and diagram your observations on the chart.
C. Observation of reproductive structures in prepared cultures and slides.
1. Examine prepared cultures of Oomycetes under a dissecting microscope for oogonia, antheridia, and oospores. Note the location of the antheridium in relation to the oogonium. How do oospores differ from oogonia?
2. Examine prepared slides using a compound microscope. Look for sporangia and sporangiophores.
a. Plasmopara viticola, cause of downy mildew of grape
b.
Albugo candida, cause of white rust of crucifers
c.
Peronospora parasitica, cause of downy mildew of broccoli
D. Observation of zoospores and their characteristics.
1. Zoospore motility: Sporangia in an aqueous preparation have been placed in water, chilled, and then warmed to induce the formation of motile zoospores. Observe the release of zoospores with a dissecting microscope or videoscope.
2. Attraction of zoospores to plant roots: Place the roots of a pea or alfalfa seedling into an aqueous suspension of
Aphanomyces or
Pythium zoospores in a petri dish, and let stand for 5-10 minutes. Observe the suspension with a dissecting microscope or videoscope for the accumulation of zoospores around the root zone of elongation.
LAB SHEETS
[TO BE PRINTED AND FILLED OUT BY STUDENTS]
OBSERVATIONS
A. Disease specimens: Record and diagram your observations of diseased plants.
 Click here for an editable and printable copy of the PROCEDURES, OBSERVATIONS, and CONCLUSIONS and QUESTIONS sections.
Click here for an editable and printable copy of the PROCEDURES, OBSERVATIONS, and CONCLUSIONS and QUESTIONS sections.
B. Diagram your observations from the prepared cultures and slides:
1. Sporangia and zoospores (include vesicles if observed)
2. Oogonia and antheridia
3. Oospores
C. 1. Record your observations of zoospores:
a. as they were released from sporangia or vesicles.
b. as they reacted to plant roots in water.
C. 2. What characteristics of zoospores did you observe?
CONCLUSIONS and QUESTIONS
1. a. How may environment play a role in the type of germination
of sporangia?
b. How does the type of sporangial germination relate to the mechanism by which spores are dispersed in the environment?
2. What fungal structure differentiates species of
Pythium from species of
Phytophthora? What is the location of this structure and its role in the
Pythium life cycle?
3. How can wet, poorly drained soil contribute to root rots caused by
Pythium and
Phytophthora?
4. In the U.S. and Europe, sexual reproduction in
Phytophthora
infestans, the cause of late blight of potato and tomato, was for many years absent because only one mating type was present in the pathogen population. Recently, however, both mating types have been found. If you suspected that both mating types were present in your potato field:
a. Where would you look to determine if sexual reproduction was occurring?
b. What fungal structure would be evidence of sexual reproduction?
