Brooks, F.E. 2002. Brown root rot. The Plant Health Instructor. DOI: 10.1094/PHI-I-2002-0923-01
Updated 2013.
Brown root rot
Phellinus noxius
Broad host range including many tropical forest, plantation and landscape trees, and woody shrubs.
Author
Fred Brooks
University of Hawaii at Manoa; formerly of the American Samoa Community College Land Grant Program

Mycelial crust of Phellinus noxius growing on a
multi-stemmed tree in the rainforest. (Courtesy F. Brooks)
Symptoms and signs
Symptoms of brown root rot disease are similar to those caused by many root rot pathogens: slow plant growth, yellowing and wilting of leaves, branch dieback, and plant death (Figure 2). Tree decline may be fast or slow, possibly depending on the number of roots affected or the location of the decay. The aboveground symptoms are caused by a root and butt rot that hinders uptake and transport of water and nutrients from the soil. Fallen trees with visible root rot are another general indication of the disease (Figure 3). Although dead wood is initially discolored reddish brown, it later becomes white, dry, and crumbly (Figure 4).
 |
 |
| Figure 2 |
Figure 3 |
 |
 |
| Figure 4 |
Figure 5 |
 |
 |
| Figure 6 |
Figure 7 |
Signs of the pathogen, unlike the symptoms, are distinctive for this disease. Phellinus noxius forms a thick, medium brown to black crust of mycelium around infected roots and, under humid conditions, around lower stems (Figure 5), Encrusted roots, often with soil and stones stuck to them, gave the disease its name. The leading edge of the crust is often creamy white, glistens with drops of clear, brownish exudate, and is usually noticeable even in the dark understory of the rainforest (Figure 6). Patches of white mycelium are present between the bark and sapwood (Figure 7). As colonization progresses, the white, soft, crumbly wood becomes laced with reddish strands of fungal hyphae that turn black with age (Figure 8). The sterile upper surface of sporocarps, or fruiting bodies, is first a velvety light brown, then a hard, uneven, dark reddish brown to black (Figure 9), The fertile pore surface is yellowish brown to gray brown (Figure 10).
 |
 |
| Figure 8 |
Figure 9 |
 |
| Figure 10 |
Pathogen Biology
Phellinus noxius, in the class Basidiomycetes, is a facultative parasite that gets its nutrients from dead and dying plant tissue. In contrast, obligate parasites like rust or powdery mildew fungi, need living hosts for their nutrient supply. The mycelium of this tropical plant pathogen grows best at 25 to 30°C (77 to 86°F); it does not grow at temperatures below 4°C (39°F) or above 40°C (104°F). Colonies of P. noxius grown in the laboratory have distinctive raised brown and white plaques.
The mycelium of P. noxius produces enzymes that break down the middle lamella and cell walls of the plant. This process supplies nutrients to the mycelium and allows it to grow deeper into the wood. It is sometimes called a white rot fungus (Figure 4) because it degrades lignin, a complex molecule that gives wood much of its strength and brown color. Brown rot fungi cannot dissolve lignin and the wood retains its brown color. Like brown rot fungi, P. noxius can also break down colorless polysaccharides, but not as quickly or as efficiently. The mycelium of P. noxius also forms arthrospores, trichocysts, microhyphae, and hyphae with extracellular sheaths. These structures are found where wood is being decomposed but their exact function is not yet known.
Sexual Reproduction
Basidiospores of P. noxius contain one nucleus (monokaryotic) and are haploid. When haploid hyphae (n) of different mating types touch each other, they can fuse (plasmogamy) to create a dikaryotic mycelium with cells containing two haploid nuclei (n + n). The dikaryotic mycelium is the most common form of this fungus in nature. The hyphae can be modified—swelling, thickening, or sticking together—to produce a mycelial crust or sporocarps. The latter often start as small round patches on stems of dead trees (Figure 11). Patches may continue to grow flat against the wood (effused, Figure 12), grow out into a shelf-like conk (reflexed, Figure 13), or a combination of both (effused-reflexed, Figure 14). The upper surface of reflexed and effused-reflexed sporocarps is sterile. The lower surface, covered with small pores, is fertile. The pores are lined with basidia, whose two nuclei fuse (karyogamy) and undergo meiosis, producing four haploid basidiospores (Figure 15). During wet weather basidiospores are released and spread by the wind.
 |
 |
| Figure 11 |
Figure 12 |
 |
 |
| Figure 13 |
Figure 14 |
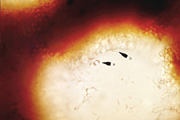 |
| Figure 15 |
Phellinus noxius sporocarps are sometimes confused with those of other Phellinus species. Some of these species have short, reddish-brown, cone-shaped cells called hymenial setae growing into their pores (Figure 16), but Phellinus noxius does not (Figure 17).
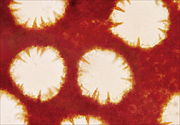 |
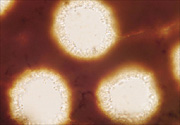 |
| Figure 16 |
Figure 17 |
Disease Cycle and Epidemiology
Epidemiology
Phellinus noxius is present in native tropical forests and plantations on infected roots and stumps and in woody debris. It does not form survival structures, such as sclerotia or resting spores, but may persist in dead roots and colonized wood for many years. Centers of disease radiate outward as roots of healthy trees come in contact with roots of diseased trees. Plants of all ages are susceptible. A mycelial crust forms around infected roots, secreting wood-rotting enzymes as it moves up the roots to the stem. Crusts 0.5 to 1.0 cm (0.2 to 0.4 in.) thick usually extend 1 to 2 m (3 to 6 ft) up the stem, though crusts almost 5 m (15 ft) high have been measured (Figure 18). If the tree does not die from severe root rot, it is killed when the crust surrounds the stem and the underlying mycelium destroys the living sapwood. Sporocarps occasionally develop on standing dead trees, stumps, or trees blown down following severe root decay. Infection of freshly cut stumps or wounds by windborne basidiospores has been demonstrated experimentally. Though basidiospores may be responsible for some long distance dispersal, the most important means of disease spread is root-to-root contact.
 Figure 18
Figure 18Disease Management
Tropical forests to be cleared for planting must be carefully surveyed for signs or symptoms of brown root rot. Planting in or near infected areas should be avoided, if possible. Partial success in managing affected areas has been obtained by pushing diseased trees and stumps into piles with a tractor and burning them. Some infected roots and debris will still remain in the soil, however, so newly planted trees that develop symptoms should be removed, roots and all, as quickly as possible. Leaving land fallow for several years or planting annual crops with vigorous root systems to break down remaining debris may offer the best approach. These methods are seldom practiced, however, due to economic considerations.
Decreasing root contact reduces spread of the fungus. This is especially important in monocultures (see Dutch elm disease lesson), as soilborne pathogens usually spread faster in single-species plantings than in mixed-species plantings. Trees should be planted close enough together for acceptable yield but far enough apart to minimize root contact. Removing large diameter trees may reduce disease spread by stopping the growth of their extensive root systems.
Chemical control is not economical on a large scale and tree varieties of important crop species resistant to Phellinus noxius have not been developed. Future solutions may be found in genetic engineering or classic biological control methods.
Significance
The spread of root diseases in native rainforests is usually restrained by differences in local conditions, such as soil type, plant density and diversity, and the balance of microorganisms in the soil. Clearing forests upsets these natural restraints. When E. J. Corner described Fomes noxius (now Phellinus noxius) as a new species in 1932, he said it was usually found in cleared or disturbed areas. Recent surveys tend to support this. Since the beginning of the 20th century, many plantations of rubber, tea, cocoa, coffee, oil palm, and mahogany established on cleared forests sites have been damaged or destroyed by P. noxius.
As growing human populations in tropical countries convert agricultural land to other uses, remaining native forests are cleared for plantations, large-scale farms, and subsistence agriculture (Figure 19). Tropical soils are quickly depleted of nutrients, however, and may be lost to erosion during heavy monsoon rains. Further, the presence of fungal pathogens such as P. noxius can destroy even the most carefully managed project. Some scientists are also concerned with loss of genetic diversity, both by the direct killing of endangered species and by destruction of their habitats. An estimated 50 to 70 percent of the world's species live in tropical forests and these forests are being cleared at the rate of about 2 percent per year (Figure 20).

Figure 19 |

Figure 20 |
Selected References
Adaskaveg, J. E. and J. M. Ogawa. 1990. Wood decay pathology of fruit and nut trees in California. Plant Disease 74: 341-352.
Ann, P-J, T-T. Chang, and W-H. Ko. 2002. Phellinus noxius brown root rot of fruit and ornamental trees in Taiwan. Plant Disease 86:820-826.
Bloomberg, W. J. 1990. Effect of stand conditions on advance of Phellinus weirii in Douglas-fir plantations. Phytopathology 80: 553-559.
Bolland, L. 1984. Phellinus noxius: cause of a significant root-rot in Queensland hoop pine plantations. Australian Forestry 47: 2-10.
Brooks, F. E. 2002. Brown root rot disease in American Samoa's tropical rain forests. Pacific Science 56: 377-387.
Chang, T. T. 1996. Survival of Phellinus noxius in soil and in the roots of dead host plants. Phytopathology 86: 272-276.
Chang, T. T. and W. W. Yang. 1998. Phellinus noxius in Taiwan: distribution, host plants and the pH and texture of the rhizosphere soils of infected hosts. Mycological Research 102: 1085-1088.
Corner, E. J. H. 1932. The identification of the brown-root fungus. The Gardens' Bulletin, Straits Settlements, Vol. V, No. 12: 317-350.
Nandris, D., M. Nicole, and J. P. Geiger, 1987. Root rot disease of rubber trees. Plant Disease 71: 298-306.
Pegler, D. N. and J. M. Waterston. 1968. Phellinus noxius, C. M. I. Descriptions of Pathogenic Fungi and Bacteria, No. 195. Commonwealth Mycological Institute, Kew, U. K.
