Blackleg
Leptosphaeria maculans (teleomorph) Phoma lingam (anamorph)
Oilseed rape including canola (and a number of other brassica or crucifer crops such as cabbage)
Although blackleg is an important disease of cabbage and some other brassica (crucifer) crops including broccoli, Brussels sprouts, cauliflower, rutabaga, and turnip, it is the most important disease of oilseed rape, including canola, with a potential to devastate the crop. Oilseeds are one of the most valuable agricultural crops in world trade.

A canola field in full bloom. (Courtesy G. Ash)
Symptoms and Signs
The first obvious symptom of blackleg of oilseed rape (rapeseed) (Brassica napus, B. rapa, and some other Brassica spp.) caused by the fungus Leptosphaeria maculans, is the appearance of gray-green to ash-gray lesions on the lower leaves (Figure 2). The presence of small, black pycnidia at the edge or scattered across the blackleg lesions distinguishes them from lesions caused by another common foliar pathogen, Alternaria brassicae (Figure 3). Alternaria lesions have no pycnidia and typically contain concentric rings. Tissue in a lesion may dry, crack, and fall out, making identification difficult (Figure 4). Blackleg lesions from multiple infections may coalesce (Figure 5). The lesions often expand down leaf veins towards the base of the leaf. In severe epidemics, lesions also can be found on the stems and pods of oilseed rape plants.
|

Figure 2
|

Figure 3
|
|

Figure 4
|

Figure 5
|
Basal stem lesions are the most damaging. When these occur in the seedling or rosette phases of growth, symptoms resemble damping-off or cut-worm damage (Figure 6). In older plants, the more typical canker symptom (Figures 7 and 8) leads to premature ripening or lodging (falling over) of the crop (Figures 9, 10, and 11).

Figure 6 |

Figure 7 |

Figure 8 |

Figure 9 |

Figure 10 |

Figure 11 |
Pathogen Biology
Leptosphaeria maculans was first described by Tode in 1791, who reported blackleg on dead cabbage stems and named the pathogen Sphaeria lingam. In 1849, Desmazieres found the same fungus on living plants and transferred it to the genus Phoma (Phoma lingam). Smith, in 1956, first discovered the perfect (sexual) stage of P. lingam and named it Leptosphaeria napi. In 1964, Smith, Sutton, Boerema and van Kesteren changed the name to L. maculans.
Sexual reproduction
Leptosphaeria maculans produces pseudothecia on the decaying stems and leaves of infected plants (Figure 12). These black, globose fruiting bodies with protruding ostioles 300-500µm in diameter, are immersed in the host tissue, and become erumpent when mature (Figure 13). The pseudothecia contain cylindrical to clavate asci. Eight ascospores are encapsulated within each bitunicate ascus (Figure 14). The ascospores are biseriate (in two rows) and have a cylindrical to ellipsoidal shape and mostly rounded ends (Figure 15). They are a yellow- brown color, with 5 transverse septa, and guttulate (containing small oil droplets). The fungus is heterothallic, i.e. two different mating types must be present for sexual reproduction to occur.

Figure 12 |

Figure 13 |

Figure 14 |
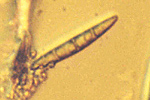
Figure 15 |
Asexual reproduction
Two types of pycnidia are found in diseased tissues. Type I (sclerotioid form) are immersed in the host tissue and become exposed in groups. The pycnidia lack a definite shape, have narrow ostioles, and have walls composed of several layers of thick-walled sclerenchymatous cells. Type II pycnidia are globose and black with a wall composed of several layers of cells. Only the cells in the outer layer are thick-walled. Pycnidiospores (conidia) are unicellular, hyaline (colorless) spores, cylindrical and straight, although a few may be curved. There is one guttule (oil droplet) at each end of the spore. These asexual spores are produced in pink to amethyst ooze under moist conditions (Figure 16).
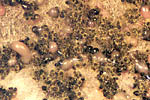
Figure 16 |
Disease Cycle and Epidemiology
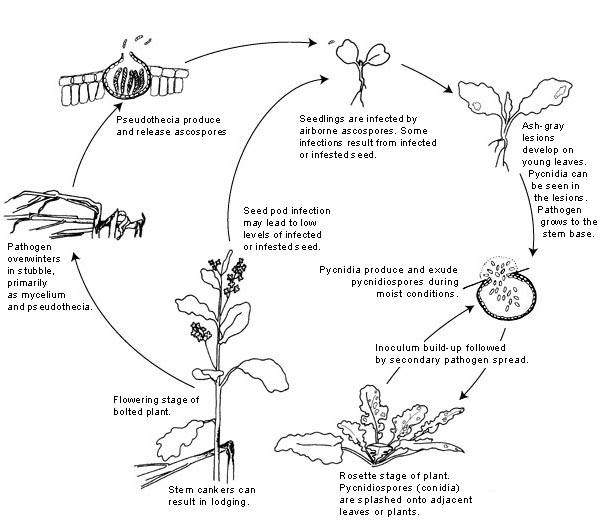
Disease cycle
Leptosphaeria maculans survives the intercrop period as mycelium and pseudothecia in crop residues. In Canada, leaf tissue does not persist long enough to allow pseudothecia to develop, but pseudothecia do form on basal stem tissue. Upon maturity, the pseudothecia produce ascospores.
Ascospores of the fungus are released after rainfall when temperatures are between 8-12ºC/46-54ºF. These spores can be wind-dispersed for hundreds of meters (yards). Pycnidia can and do overwinter readily in stubble, but because pycnidiospores are not airborne to any significant extent, they are of minor importance in initiating the first cycle of disease.
Ascospores germinate in the presence of free water from 4-28ºC (40-82ºF). Penetration is through stomates. The pathogen also may be seedborne. Seeds may be infected and/or infested by the pathogen. Infected seeds can give rise to infected seedlings, but levels of seed contamination are always very low. Primary infections usually occur on the cotyledons or basal rosette leaves of the plant. Wet weather favors these primary infections.
The fungus invades the intercellular spaces between the palisade and epidermal layers of the leaf. This symptomless biotrophic phase is followed by invasion of the mesophyll with the resultant death of cells and the appearance of gray-green lesions. The hyphae continue to ramify through the leaf tissue until they reach a leaf vein. The fungus then colonizes the cortex and/or xylem parenchyma of the petiole. At the junction of the petiole and the stem, the fungus invades the stem cortex where it causes a canker. It is at this point that stem resistance is expressed and determines the ability of the disease to proceed to the damaging stem canker phase. Stem cankers form when the plants bolt (produce an upright stem from the rosette on which the flowers form). Stem cankers develop most quickly at 20-24ºC/68-75ºF and are most severe under stress conditions such as mechanical, insect, or herbicide injury.
Pycnidiospores (conidia) are released from the pycnidia under moist conditions in a mucilage, a watery, sticky solution. These spores are responsible for secondary cycles of the disease, but ascospores are the more important source of inoculum because they are more infective and are airborne.
The pynidiospores are dispersed to new infection sites by rain splash. Pycnidiospores germinate more slowly than ascospores and require more than 16 hr of continuous wetness at the optimal temperature range of 20-25ºC/68-77ºF. The minimum latent period (the time from infection to the production of new inoculum) following infection by pycnidiospores is 13 days. Although secondary infections by pycnidiospores do occur, most losses are due to primary infections of leaves by ascospores that lead to basal stem cankers and eventual lodging of the plants.
Disease Management
Cultural control
Cultural practices such as field selection, stubble management, control of volunteer crop plants and other susceptible hosts such as certain weeds, and pathogen-free seed can reduce the incidence of blackleg. Because the pathogen survives on stubble, it is important to avoid planting oilseed rape in fields that have stubble from a previous susceptible crop. This requires rotating away from oilseed rape for three to four years (longer in drier climates). There should be a minimum distance of 500 meters (yards) between oilseed rape crop residue and oilseed rape crops planted in fields without a history of the disease or where a rotation is used. This distance will significantly reduce infection by windborne ascospores from stubble residues.
Stubble residues can be destroyed by burning or by deep plowing, however burning is not allowed in many areas. Incorporation of residues hastens decomposition by soil microorganisms and helps to prevent discharge of ascospores into the air. Drier regions have more carryover of crop residues from year to year because moisture is a limiting factor in decomposition.
Although the pathogen is seedborne, incidence of seed contamination is generally very low. The use of pathogen-free seed reduces initial inoculum, especially when planting in new areas or areas that have a low incidence of the disease. Most certified seed fields are inspected by scouting to determine that disease is not present rather than by direct testing of the seed. Some government labs in Canada provide a testing service to determine if seed is contaminated by the pathogen.
Fungicides
Fungicides have been used to manage blackleg, either as seed or fertilizer dressings or as foliar applications. In Australia, fungicide is used to coat super phosphate fertilizer and then to the crop in a furrow application. Application of fungicides to seeds is usually more economically feasible than foliar applications. Seed treatments with benzimidazole, dicarboximide and morpholine fungicides can almost eliminate seed transmission of the pathogen. To prevent the introduction of the pathogen to noninfested areas, the planting of certified seed treated with a fungicide is recommended. Early control of blackleg reduces the likelihood of the damaging stem canker phase. Successful control through foliar applications, primarily to seedlings to prevent initial infections, has been reported using benzimidazole, carboxamide, and dicarboxamide fungicides. Application of fungicides or herbicides to stubble for suppression of pseudothecia formation has been tested experimentally, but this approach is not used widely because of economics, erratic performance, and the planting of disease-resistant cultivars in many areas.
Genetic resistance
Genetic resistance is the most important and sustainable means of controlling blackleg. The genetic basis of much of the resistance, however, is not well understood in oilseed rape. Genetic strains of the fungus that vary in host range and in virulence on a particular host species have been observed. Some resistant cultivars are available, but cultivar choice is dependent on which strains of the fungus are present in a particular area.
Significance
Oilseeds are one of the most valuable agricultural crops in world trade. Oilseed rape (Brassica napus, B. rapa, and some other Brassica species) ranks third in the world among the oilseed crops, and production is increasing faster than for any other. Oilseed rape was first cultivated in Asia and the Mediterranean and was used as a source of cooking and lighting oil. Canola (Figure 1) is a type of oilseed rape defined by erucic acid levels lower than 2% in the oil (so the oil is edible) and less than 30 mol/g of glucosinolates in the meal (so it can be fed to livestock).

Figure 1 |
Blackleg is the most important disease of oilseed rape, including canola, with a potential to devastate the crop. It also is an important disease of cabbage and some other brassica (crucifer) crops including broccoli, Brussels sprouts, cauliflower, rutabaga, and turnip.
Yield losses of up to 100% due to blackleg have been recorded in oilseed rape. In Australia in the 1970s, the rapeseed industry was completely destroyed by epidemics of blackleg. Blackleg on canola crops in the U.S. first occurred in Kentucky in 1989, about three years after the crop was introduced. Some fields had 100% losses with nearly every plant lodging and dying (Figure 10).

Figure 10 |
Blackleg is currently the most important disease of canola in western Canada. The disease first appeared in 1975, and the first widespread occurrences of severe basal stem canker were recorded in central Saskatchewan in 1982. The disease quickly spread to the neighboring provinces and is now endemic in most canola growing regions of the western Canadian prairies.
Selected References
Symposium on the Biology and Control of Leptosphaeria maculans:
Williams, P.H. 1992. Biology of Leptosphaeria maculans. Can. J. Plant Path. 14:30-35.
Gugel, R.K. and G.A. Petrie. 1992 History, occurrence, impact, and control of blackleg of rapeseed. Can. J. Plant Path. 14:36-45.
Hall, R. 1992. Epidemiology of blackleg of oilseed rape. Can. J. Plant Path. 14: 46-55.
Rimmer, S.R. and C.G. J. vandenBerg.1992. Resistance of oilseed Brassica spp.to blackleg caused by Leptosphaeria maculans. Can. J. Plant Path. 14:56-66.
Soledade, M., C. Pedras, and G. Seguin-Swartz.1992. The blackleg fungus: phytotoxins and phytoalexins. Can. J. Plant Path. 14:67-75.
Hammond, K.E and B.G. Lewis. 1986. The timing and sequence of events leading to stem canker disease in populations of Brassica napus var. olefera in the field. Plant Pathol. 35:551-564.
McGee, D.C and G.A. Petrie.1978. Variability of Leptosphaeria maculans in relation to blackleg of oilseed rape. Phytopathology 68: 625-630.
Salisbury, P.A., D.J. Ballinger, N. Wratten, K.M. Plummer, and B.J. Howlett.1995. Blackleg disease on oilseed Brassica in Australia: a review. Austral. J. Exp. Agr. Animal Husb. 35:665-672.
Shaahidi, F. 1990. Canola and Rapeseed. Production, Chemistry, nutrition and Processing Technology. Van Nostrand Reinhold, New York, NY.
Williams, P.H. and B.D.L. Fitt. 1999. Differentiating A and B groups of Leptosphaeria maculans, causal agent of stem canker (blackleg) of oilseed rape. Plant Pathol. 48: 161-175.
