Apple scab
Venturia inaequalis
Apples and flowering crabapples (Malus spp.), hawthorn (Crataegus spp.), mountain ash (Sorbus spp.), firethorn (Pyracantha spp.), and loquat (Eriobotrya japonica). Pear (Pyrus spp.) is infected by a related fungus, Venturia pirina, which causes similar symptoms. However, the apple scab pathogen will not infect pear, and the pear scab pathogen will not infect apple.

Figure 1. Typical “scabby” lesions are diagnostic symptoms
of advanced apple scab on fruit.
Apple scab occurs everywhere in the world where apples are grown and results in more losses than any other apple disease. It is most serious in areas that have cool, wet spring weather and may not be economically important in warm and/or dry climates.
Symptoms and Signs
Apple scab results in symptoms on most upper plant parts, most notably leaves and fruit. Petioles, flowers, sepals, pedicels, young shoots, and bud scales can also become infected.
Apple leaf and fruit symptoms
Apple scab infections are initiated in early spring on emerging and young leaves. Early lesions appear 10 days later as lighter green areas compared to the surrounding leaf tissue. Lesions increase in size and become olive-colored and velvety as a result of asexual spore production (conidia) (Figures 2 and 3). Scab lesions that form on young leaves may expand to more than 1 cm in diameter. Ontogenetic resistance of older leaves, however, usually results in smaller lesions or no visible symptoms. Affected tissues eventually may become distorted and puckered, and leaf lesions become cracked and torn. Severely infected leaves drop from trees. Two to three consecutive defoliation events can weaken trees, resulting in a greater susceptibility to other stresses such as freeze damage, insect injury, and other diseases.
Fruit lesions are generally blistered and "scabby" in appearance, with a distinct margin (Figures 4 and 5). The earliest noticeable symptom on fruit is water-soaked areas that rapidly develops into velvety, green to olive-brown lesions. Infections of young fruit cause fruit distortion as healthy tissue continues to grow (Figure 5). Severely infected fruit often drop prematurely from trees.

Figure 2. Early lesions are olive-colored (A) and velvety
with no distinct margins (B). |

Figure 3. Affected leaves
become distorted and puckered. |

Figure 4. Fruit lesions appear blistered and scabby
(A) with distinct margins (B). |

Figure 5. Infections of young
fruit cause distortion as fruit mature. |
Apple blossoms
Blossom infections usually develop into small, dark green lesions at the base of flowers, on sepals, and on stem pedicels before and during bloom. Developing fruit may drop from infected pedicels, resulting in lower fruit yield. Reduced and late-return bloom can result from infections that had occurred in preceding years.
Pathogen Biology
Venturia inaequalis is an ascomycete fungus; it produces sexual spores (ascospores) in a sac-like structure called an ascus (plural asci). The mycelium of V. inaequalis is septate, and the nuclei are haploid.
Sexual Reproduction
Venturia inaequalis overwinters in fallen leaf and fruit debris as pseudothecial initials. These structures emerge in early spring at approximately the same time as host plants break dormancy.
Mating between the two different mating types takes place in debris, and both mating types must be present in order for sexual reproduction to be initiated. Mating consists of the fusion of a male organ (antheridium) formed from a hyphal tip of one mate to a female receptive hypha (trichogyne) from the opposite mate. The trichogyne is attached to a coil of hyphae called the pseudothecial initial. During fertilization, nuclei pass from the antheridium through the trichogyne into a cell at the base of the pseudothecial initial.
After fertilization, the pseudothecial initial develops into a pseudothecium (Figure 6), a cavity located within a dense mat of fungal mycelia called a stroma. Inside this cavity, asci and ascospores are formed (Figure 7). The very brief diploid stage in the life cycle of V. inaequalis occurs within the pseudothecium in single hyphal cells (croziers), which give rise to the haploid ascospores following meiosis. Asci are elongated, sac-like structures, each of which contains eight ascospores in a linear arrangement (Figure 8). The ascospores are brown, two-celled spores, and have a characteristic "footprint" shape (Figure 9). The shape of ascospores inspired the Latin name for apple scab, "inaequalis", which refers to the unequal size of the cells. Ascospores measure between 5 and 7 µm wide and between 11 and 15 µm long.
During wet conditions, mature pseudothecia swell and protrude from the surface of fallen leaves (Figure 10). Ascospores are released and carried to blossoms and leaves by rain and wind. There is only one cycle of ascospore production and infection per season.
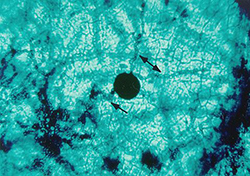
Figure 6. Pseudothecial initial develops into
a pseudothecium. Arrows show hyphae,
which may represent the two mating types. |
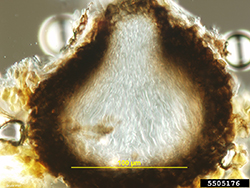
Figure 7. Asci and ascospores form inside a
pseudothecium. Asci and ascospores may
be stained (not shown) for enhanced viewing. |
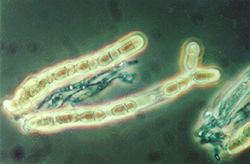
Figure 8. Asci of Venturia inaequalis containing eight ascospores. Arrows highlight individual ascospores. |
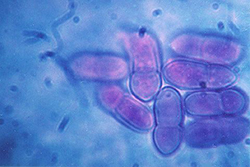
Figure 9. Two-celled ascospores of Venturia inaequalis are footprint-shaped. |
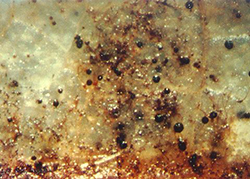
Figure 10. View of a fallen apple leaf, showing the mature
pseudothecia of Venturia inaequalis protruding above the
surface ready to release ascospores. |
Asexual Reproduction
V. inaequalis reproduces asexually by spores called conidia. Conidia are single-celled, uninucleate, and narrower at one end than the other (Figure 11). In mass, conidia appear brown or olive, but they are lighter when viewed individually under a microscope. Conidia measure between 6 and 12 µm wide and 12 and 22 µm long and are produced by specialized short hyphae called conidiophores. Conidiophores are formed on a dense mat of mycelia that pushes up through and ruptures the leaf cuticle (Figure 12). It is this mass of conidia and conidiophores that causes the velvety appearance of young scab lesions.
Conidia are produced nine to thirty days after initial leaf infection, depending upon temperature. They are disseminated by wind and by wind-driven rain. Both ascospores and conidia require a period of wetness in order to germinate. The germination hypha penetrates the cuticle and establishes a new infection. There can be many cycles of conidial production and infection within a single growing season.
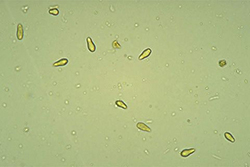
Figure 11. Conidia are asexual,
single-celled spores
|
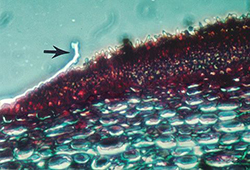
Figure 12. Cross section through a leaf infected with apple scab. Arrow indicates the leaf cuticle, which has been ruptured and pushed back by the mass of erupting conidia and conidiophores. |
Disease Cycle and Epidemiology
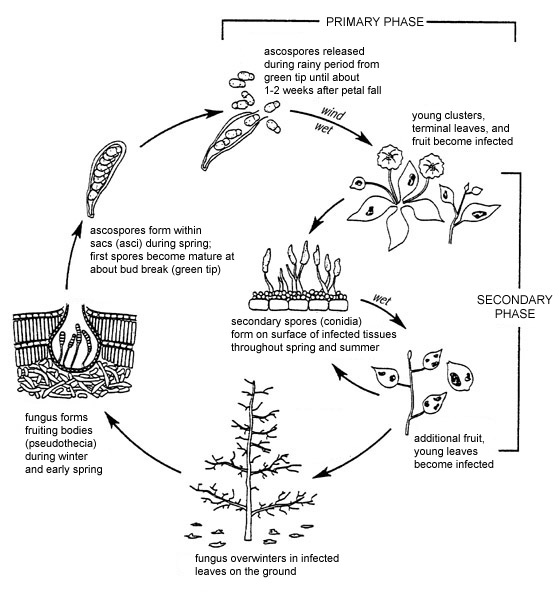
Figure 13. Disease cycle of apple scab.
Disease Cycle
During the growing season, the apple scab fungus is found only in the area between the host cuticle and the epidermis. Once infected leaves fall from trees and die, the fungal mycelia colonize them completely. Venturia inaequalis usually overwinters in fallen leaves as mycelia and pseudothecial initials. In milder climates, the fungus may overwinter in infected buds and produce conidia that serve as primary inoculum. In leaf debris, most pseudothecial initials form within a month after leaves fall, and then enter a period of dormancy.
In early spring, mating (sexual reproduction) takes place in leaf debris between two mating types (as described above). In response to rain events, asci expand through the ostiole, a hole at the top of the pseudothecia. Ascospores are forcibly discharged and spread by wind and by wind-driven rain. Sufficient moisture must be present for ascospores to infect blossoms and young leaves. In most years, ascospore dissemination coincides with the several-week period between budbreak and the end of bloom.
Lesions caused by ascospores (primary infections) produce asexual conidia within 9 days and up to 30 days later. Conidia are dispersed to healthy leaves and developing fruit, where they establish secondary infections. Up to 100,000 conidia can be produced by a single lesion. Lesions from primary or secondary infections expand at a rate that is determined partly by temperature and partly by characteristics of the host tissue, including genotype and age. Lesion expansion, in turn, affects the rate at which new spores are produced. During cooler conditions, or on more resistant cultivars, lesions expand more slowly and may be smaller in size. As a result, the number of secondary cycles will be fewer.
Infection and spore production are dependent upon available moisture. Infection by ascospores and conidia is highly dependent upon how long the leaves or fruit stay wet, as well as on the average temperature. The Mills table relates leaf wetness duration and temperature to determine the likelihood that conidial infection will occur (Figure 13). For example, at an average temperature of 18°C (65°F), light infection will result if leaves remain wet for 9 h. Lesions can produce conidia after 9 days if the temperature averages 18°C (65°F), but not until 17 days if the temperatures are lower, averaging only 8°C (49°F). The Mills table continues to be revised as more data are gathered from different regions, and modifications by A.L. Jones comprise the most commonly accepted version.
Table 1. Wetting period (in hours) required for apple scab infection at different air temperatures, and time required for development of conidia by lesions at different air temperatures.
| Wetting period (HOURS) |
| Average Temperature (F) |
Average Temperature (C) |
Light Infection |
Moderate Infection |
Heavy Infection |
Incubation Period (days) |
| 78 |
25.6 |
13 |
17 |
26 |
... |
| 77 |
25.0 |
11 |
14 |
21 |
... |
| 76 |
24.4 |
9.5 |
12 |
19 |
... |
| 63-75 |
17.2-23.9 |
9 |
12 |
18 |
9 |
| 62 |
16.7 |
9 |
12 |
19 |
10 |
| 61 |
16.1 |
9 |
13 |
20 |
10 |
| 60 |
15.6 |
9.5 |
13 |
20 |
11 |
| 59 |
15.0 |
10 |
13 |
21 |
12 |
| 58 |
14.4 |
10 |
14 |
21 |
12 |
| 57 |
13.9 |
10 |
14 |
22 |
13 |
| 56 |
13.3 |
11 |
15 |
22 |
13 |
| 55 |
12.8 |
11 |
16 |
24 |
14 |
| 54 |
12.2 |
11.5 |
16 |
24 |
14 |
| 53 |
11.7 |
12 |
17 |
25 |
15 |
| 52 |
11.1 |
12 |
18 |
26 |
15 |
| 51 |
10.6 |
13 |
18 |
27 |
16 |
| 50 |
10.0 |
14 |
19 |
29 |
16 |
| 49 |
9.4 |
14.5 |
20 |
30 |
17 |
| 48 |
8.9 |
15 |
20 |
30 |
17 |
| 47 |
8.3 |
17 |
23 |
35 |
17 |
| 46 |
7.8 |
19 |
25 |
38 |
17 |
| 45 |
7.2 |
20 |
27 |
41 |
17 |
| 44 |
6.6 |
22 |
30 |
45 |
17 |
| 43 |
6.1 |
25 |
34 |
51 |
17 |
| 42 |
5.5 |
30 |
40 |
60 |
17 |
Disease Management
Chemical Management
Management of apple scab is focused on the prevention of primary infection by ascospores. This is because early infection by ascospores may result in poor fruit set and will result in more secondary inoculum throughout the season. Fungicide applications are therefore timed to coincide with the spring release of ascospores (between bud break and petal fall). Maturing plant tissue is somewhat resistant to infections, and scab-targeted fungicides are reduced or sometimes eliminated from spray schedules unless conditions are conducive for disease (wet weather, susceptible cultivars, and/or high disease pressure). Later-season fungicide sprays are often targeted toward other fungal diseases, but also may be effective against secondary inoculum. Disease severity and distribution varies, so growers should utilize local university sources when developing spray schedules.

Figure 14. Contact fungicides require complete, uniform coverage. Leaf appears to
be completely covered with residue. |

Figure 15. An airblast sprayer being used to apply fungicides in a commercial orchard.
|
Various fungicide chemistries are available for management of apple scab. Most products can be classified as contact or systemic. Contact fungicides form an external barrier on plant surfaces. They are typically not rainfast (they wash off readily with rain) and do not translocate through plant tissue. Systemic fungicides, in contrast, penetrate epidermal tissue and can translocate within plant tissue. They are more rainfast than contact fungicides, and they usually have longer reapplication intervals.
Both contact and systemic fungicides are applied before infection to prevent fungal spores from germinating or penetrating host tissue. Fungicides do not cure disease, even though some older recommendations may refer to systemic fungicides as curative products. Thorough spray coverage and uniform deposition are essential, especially for contact fungicides (Figure 14). To ensure coverage of newly emerging tissues and due to breakdown of active ingredients, fungicides must be re-applied on a regular schedule. This application schedule is dependent upon product formulation and systemic properties; consult product label for individual mrecommendations. In commercial orchards, airblast sprayers (Figure 15) are typically used to apply fungicides to ensure sufficient coverage.
Fungicides are classified according to their mode of action. The Fungicide Resistance Action Committee (FRAC) has categorized fungicides according to their mode of action and, accordingly, their risk for resistance development. Many newer fungicides have a high risk for resistance, so it is important to rotate between FRAC groups to prevent development of resistant populations.
Timing of fungicide applications is critical for effective scab management. When fungicides are combined with cultural practices and are based on environmental conditions (e.g. prediction models such as a Mills table-based weather monitoring system, Figures 16, 17) disease is more effectively managed. Fungicide applications based on weather monitoring systems can reduce numbers of sprays and/or more accurately time applications, thereby reducing inputs and increasing grower profits. Several university programs and private industries have developed regionally-specific prediction models that utilize weather forecasts to estimate leaf wetness and temperature conditions to evaluate risk for spore release and infection.

Figure 16 |

Figure 17 |
Biological Control
There are a limited number of biological control products available for management of apple scab. To date, many of these products have below-average efficacy ratings when compared to traditional fungicides; thus, use of cultural controls and resistant cultivars are vital components of a biological control disease management program.
Genetic Resistance
Scab-resistant (or more appropriately scab-tolerant) apples and crabapples are widely available. Levels of resistance/tolerance vary with cultivar, although most scab-resistant apple cultivars carry the Vf resistance gene from M. floribunda 821. For many years, resistance resulted in reduction and/or elimination of fungicide use for scab management. However, reports of loss of host resistance were reported in Europe in 1993 and in North America in 2007.
In many areas, resistance remains intact. Risk for loss of host resistance (due to adaptability of the pathogen) is high when growers rely on resistance alone.
In all cases, an integrated management (IPM) program helps prevent resistance breakdown, as well as manage disease in orchards where resistance has been compromised. Cultural practices (improved air circulation and sanitation) are critical for reduction of inoculum. Additionally, use of fungicides (synthetic, organic, and/or biological) during primary infection periods also reduce inoculum and help break the disease cycle. Resistant and susceptible cultivars should not be planted in close proximity. Integrated use of these and other management practices help protect resistance and reduce disease pressure. Resistant or tolerant apple cultivars are gaining acceptance by both growers and consumers. Resistant cultivars are listed in Table 2.
Table 2. List of popular scab-resistant apple cultivars.

Susceptible cultivars are still widely grown due to popularity and consumer demand. Additionally, these cultivars often can be grown easily in drier regions where apple scab is not a problem. Cultivars that are highly susceptible are listed in Table 3.
Table 3. List of popular apple cultivars that are susceptible to scab.

Scab-resistant flowering crabapples provide greater bloom potential because there is no risk for defoliation (Figures 18 and 19). Many scab-resistant cultivars are available, and many new ones are being introduced. Table 4 includes examples of crabapples from the Morton Arboretum (http://www.mortonarb.org/) that exhibit excellent resistance.

Figure 18 |

Figure 19 |
Table 4. List of popular scab-resistant crabapple on
display at the Morton Arboretum near Chicago, IL..

Cultural Practices
Cultural practices should be combined with fungicides (chemical and/or biological) for an integrated pest management system. Two common cultural practices used to reduce apple scab are sanitation and increased air circulation. Sanitation eliminates inoculum during the growing season and during the overwintering season. Commercial growers use various methods to speed up leaf decomposition, thereby decreasing the amount of primary inoculum the following season. These include: applying urea to trees, just before leaf drop, applying urea to fallen leaves, and tilling fallen leaves into the soil or chopping them into small pieces. Another effective cultural practice is to lower wetness and humidity in tree canopies so that the environment becomes unfavorable for disease. Pruning and wide tree spacing can enhance air movement and allow sunlight to penetrate, which speeds up drying of leaves and fruit.
Significance
Apple scab has long been a problem on apples; symptoms of the disease can be recognized on fruit in paintings from the fifteenth and sixteenth centuries, as well as the 1824 painting by American James Peale, shown in Fig. 20.
 |
Figure 20. Fruit Still Life with Chinese Export Basket,
painted in 1824 by American James Peale (1749–1831). Note the
symptoms of scab on the apple on the table at the left side of the painting. |
The frequent historical depictions of scab-infected apples suggest that the disease was common and that the affected fruit was acceptable in earlier times. All the commonly grown apple cultivars were susceptible to the disease, and there were no chemical treatments until the late 1800s. At that time, copper- and sulfur-based fungicides served as protectants, but the treatments often caused substantial damage to the apple foliage. Even today, in spite of the highly effective chemicals and the resistant apple cultivars that are available, apple scab causes greater economic losses of apples in North and South America, Europe, and Asia than any other apple disease.
Venturia inaequalis was one of the first ascomycetes to be subjected to genetic analysis; the heritability of pathogenicity and of sexual compatibility was investigated as early as the 1930s. It has served as and continues to be a valuable tool for basic genetic research and for studies of the inheritance of pathogenicity. Among the features that make Venturia inaequalis so amenable for genetic research is the fact that it is similar to many obligate parasites that infect young tissues and live in a close association with them, without visibly harming them, for an extended period of time. Yet unlike obligate parasites, it can be cultured on artificial media, and matings can be made in vitro. Other advantages of V. inaequalis for genetic studies include its considerable diversity, with many pathotypes or races occurring in the natural population; the stability of phenotypes and genotypes in culture over many years; the fact that it is haploid, allowing the effects of alleles to be studied directly; the uninucleate conidia which give rise to colonies in which all the nuclei are genetically uniform; and the ability to isolate all eight of the ascospores from a single ascus (Figure 9). The eight spores are the result of a single meiotic event, and so segregation of traits can be studied directly rather than statistically. Furthermore, the eight ascospores are arranged in order, allowing reconstruction of the meiotic divisions and mapping of centromeres as genetic loci. Many of these advantages are shared by other Ascomycetes, but they are uniquely brought together in V. inaequalis.
Many years of study have demonstrated that the genetics of pathogenicity in V. inaequalis are not simple. There are multiple genes involved in pathogenicity to various apple species and cultivars. In several of these interactions, pathogenicity is conditioned by single genes. In some cases, there appears to be a gene-for-gene relationship between the host and the pathogen. In a gene-for-gene interaction, each gene for resistance in the host is matched by a gene for pathogenicity (or virulence) in the pathogen. Resistance will only occur if the host has the dominant allele for the resistance gene and the pathogen has the corresponding dominant allele conditioning non-pathogenicity (or avirulence); all other combinations will result in disease. Gene-for-gene interactions are found in numerous host-pathogen interactions, and are thought to be the consequence of co-evolution of the host and the pathogen.
Selected References
Beckerman, J. 2009. Managing scab-resistant apples (BP-76-W). Purdue University. Purdue Extension Education Store. Available for download from: https://edustore.purdue.edu/
Fungicide Resistance Action Committee. www.frac.info.
Gessler, C., and Pertot, I. 2012. Vf scab resistance of Malus in: Trees (2012) 26:95. Doi 10.1007/s00468-011-0618-y.
Jones, A. L., Lillevik, S. L., Fisher, P. D. and Stebbins, T. C. 1980. A microcomputer-based instrument to predict primary apple scab infection periods. Plant Dis. 64:69-72.
Merwin, I. A., Brown, S. K., Rosenberger, D. A., Cooley, D. R., and Berkett, L.P. 1994. Scab-resistant apples for the Northeastern United States: New prospects and old problems. Plant Disease 78:4-
0.
MacHardy, W. E. 1996. Apple Scab Biology, Epidemiology, and Management. APS Press St. Paul 545 pp.
MacHardy, W. E. and Gadoury, D. M. 1989. A revision of Mills’ criteria for predicting apple scab infection periods. Phytopathology 79:304-301.
Morton, V., and Staub, T. 2008. A short history of fungicides. Online. APSnet Features. Doi 10.1094/APSnetFeature-2008-0308.
Parisi, L., Lespinasse, Y., Guillaumes, J., and Kruger, J. 1993. A new race of Venturia inaequalis virulent to apples with resistance due to the Vf gene. Phytopathology 83: 533–537
.
Rosenberger, D. A. 2003. Susceptibility of new apple cultivars to common apple diseases. New York Fruit Quarterly, 11(2): 17-22.
Stensvand, A., Gadoury, D. M., Amundsen, T., Semb, L., and Seem, R. C. 1997. Ascospore release and infection of apple leaves by conidia and ascospores of Venturia inaequalis at low temperatures. Phytopathology 87:1046-1053.
Sutton, T. B., Aldwinckle, H. S., Agnello, A. M., and Walgenbach, J. F., eds. 2014. Compendium of Apple and Pear Diseases, 2nd Edition. APS Press, St. Paul 224 pp.
Travis, J.W., and J. Ritter. 2006. Scab Resistant Cultivars, Table 1-6. Penn State Tree Fruit Production Guide.
