Michael E. Ostry and Jennifer Juzwik
Research Plant Pathologists
USDA Forest Service
Northern Research Station
1561 Lindig Ave., St. Paul, MN 55108
(Corresponding author: mostry@fs.fed.us)
Ostry, M.E., and Juzwik, J. 2008. Selected Forest and Shade Tree Disease of Significance in the 20th Century. Online. APSnet Features. doi: 10.1094/APSnetFeatures-2008-0508.
Introduction
Several forest and shade tree diseases such as white pine blister rust, chestnut blight, Dutch elm disease, and sudden oak death have been the dominating forest pathology stories of the past one hundred years. However, a number of other tree diseases have dramatically affected trees in a variety of landscapes or production systems that also captured the attention of forest pathologists. Many of these continue to pose a threat to the health and value of urban and rural forest ecosystems.
Forest pathologists work in natural forests and tree plantings managed for numerous objectives ranging from planting stock production in nurseries, shelter belts to protect soil and water, bioenergy plantings, Christmas tree production, managed and unmanaged rural forests for multiple values, to urban street tree plantings. Native and exotic pathogens have had significant impacts on these endeavors, albeit often on smaller scales than, for example, pathogens such as those causing chestnut blight or Dutch elm disease.
A retrospective overview of forest pathology in the last century and directions for the future was the topic of a symposium at the annual APS meeting in 2000 and these papers were published in Phytopathology in 2003 (see resources for further reading). In this article, brief descriptions of several tree diseases that have negatively impacted urban, rural and specialty forests across large landscapes and in multiple geographic regions but that are, perhaps, not as well-known by the casual reader are offered as a further retrospective view.
Scope and Scale of Tree Pathogens and Their Impacts
Forest and tree health concerns in the 20th century were the result of several factors. In the beginning of the century much of our eastern forest lands were heavily logged, burned by intense fires, and cleared for agriculture. As time went by these lands were planted and forests reclaimed abandoned agricultural fields. Nursery production of planting stock resulted in many endemic and exotic pathogens being moved across the country. Diseases of early successional species in plantations soon became important. Later in the century land use changes, forest fragmentation and amenity planting of exotic tree species across a range of sites and habitats predisposed our forests and tree plantings to numerous damaging diseases that were not previously significant.
Native pathogens often exist in equilibrium with natural forest communities and it is widely recognized that pathogens and dead trees have critical ecological roles and contribute to sustainable, healthy forest ecosystems. Damaging disease epidemics have resulted when either non-adapted or exotic tree species were planted; trees were planted on stressful sites; management changed growing conditions or when pathogens were introduced into new ecosystems. Many forests today are being managed using different silvicultural systems than in the past in response to changing values and uses of these lands by owners and public agencies responsible for land management. Systems of intensive management, artificial regeneration, and all-aged and mixed species management can influence the incidence and severity of disease caused by native and introduced pathogens.
Selected Examples of Widely Distributed and Damaging Tree Diseases
Cylindrocladium diseases. Cylindrocladium diseases are known to occur in forest nurseries in at least 20 states of the eastern US and in Ontario and Quebec in Canada. The pathogens (primarily Cylindrocladium floridanum and C. scoparium) stunt and kill seedlings of a wide range of conifer and hardwood species (Fig. 1). While significant mortality is associated with root infections, sub-lethal infections of stems and foliage (causing stunting, chlorosis and top dieback) render affected seedlings un-saleable. In the southern US black walnut, yellow poplar and eastern white pine have been the species most severely affected. Over 50% of black walnut and yellow poplar seedlings in at least six nurseries were killed or culled over a five-year period due to Cylindrocladium diseases. The host species primarily affected in northern bare-root nurseries are black spruce, red spruce, white spruce and red pine. Although Cylindrocladium has been isolated from white pine in this northern region, Fusarium spp. and soil-related factors were found to be the cause of extensive white pine root rot.
| |

Fig. 1. Red pine (Pinus resinosa) nursery seedlings killed by Cylindrocladium floridanum (Michigan 1966). (Courtesy US Forest Service). |
|
Cylindrocladium species were first discovered in northern nurseries in the latter half of the 20th century (1950s to 1970s). Losses due to Cylindrocladium root rot were severe in many cases. In one Minnesota nursery, 80% of the black spruce killed by C. floridanum in one year was estimated to cost the nursery over $40,000. The inability to control the disease and the persistent inoculum (microsclerotia, Fig. 2) led to the nursery’s closure. In Wisconsin, 60 to 80% mortality of C. scoparium infected transplants (black and white spruce and red pine) was reported for three nurseries in the 1960s. Between 30 and 55% of black spruce transplants in six fields of black spruce in a southern Ontario nursery were killed or culled due to Cylindrocladium spp. in the early 1980s with losses estimated at $65,000 (Cdn). Out-planting of undetected, but pathogen infected, stock may also result in significant mortality within several years of plantation establishment.
|
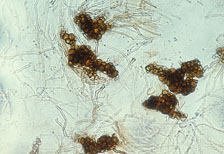
Fig. 2. Microsclerotia (overwintering propagules) of Cylindrocladium floridanum isolate from an Ontario provincial nursery (1986). (Courtesy J. Juzwik). |
|
Much success in controlling Cylindrocladium root disease has been achieved in recent decades. Important sanitation measures include restricting movement of infected stock, removing soil from tires and tillage implements of equipment used in one field before entering the next one, and root raking in affected fields following seedling lift. Cover-cropping with sorghum-sudan grass has been routinely used in Wisconsin nurseries to reduce pathogen levels in the soil. Dipping of transplant seedling roots in benomyl was also an effective control, but concerns over worker exposure to the chemical were significant. Soil fumigation (e.g., methyl bromide plus chloropicrin, dazomet, metam sodium) has proven effective in dramatically reducing viable pathogen propagules and controlling the disease. In general, Cylindrocladium root disease appears to be successfully managed in US bare-root forest nurseries.
Lophodermium needle cast. In spring 1966, red and Scots pine seedlings in nursery beds turned brown in 14 Michigan, Wisconsin and Minnesota nurseries from an unknown cause (Fig. 3). Millions of dead trees were buried in dumps or burned resulting in major losses of nursery stock. In 1968, the cause of this epidemic was determined to be the fungus Lophodermium pinastri (later correctly identified as L. seditiosum), the first report of this fungus as a major pathogen of red pine. During the early years of the epidemic, infected seedlings showing no symptoms were inadvertently shipped and planted in the field, widely spreading the pathogen. Damage to red and Scots pine seedlings in the field and in over 35 nurseries in nine states and two Canadian provinces were reported by 1974. In addition, by 1974 outbreaks of Lophodermium needle cast were occurring in Scots pine Christmas tree plantations in 16 states and one Canadian province. Entire plantations of the most popular, and most susceptible Scots pine varieties planted in North America for Christmas tree production turned brown causing major losses for growers as they were unable to sell the affected trees (Fig. 4). Control strategies, including the use of chemicals, cultural practices and use of resistant varieties, were developed and put into use for nurseries and Christmas tree plantations in the early 1970s. As a result, healthy seedlings were again being produced. Timely action by teams of researchers and growers were able to prevent further catastrophic losses for seedling and Christmas tree producers in the United States and Canada.
 |
|

|
|
Fig. 3. Red pine (Pinus resinosa) nursery seedlings killed by Lophodermium seditiosum (seedlings in foreground were protected with a fungicide) (Wisconsin 1969). (Courtesy US Forest Service). |
|
Fig. 4. Scotch pine (Pinus sylvestris) Christmas trees damaged by Lophodermium seditiosum (Michigan 1970). (Courtesy US Forest Service). |
Swiss needle cast. Phaeocryptopus gaeumannii, an endemic pathogen of Douglas-fir in the Pacific Northwest, can cause a damaging disease where Douglas-fir has been planted outside of its natural range in closely-spaced plantations established for fiber or Christmas tree production (Fig. 5). Premature needle cast results in reduced growth rates and unmerchantable trees (Fig. 6). Volume growth loss of over 25% has been estimated of coastal Douglas fir with over 207,000 acres affected in Oregon. The disease is called Swiss needle cast because it was first described on exotic Douglas-fir in Switzerland where infected, imported seedlings were most likely planted.
 |
|
 |
|
|
Fig. 5. Douglas-fir (Pseudotsuga menziesii) Christmas tree damaged by Swiss needle cast caused by Phaeocryptopus gaeumannii (Wisconsin 1980). (Courtesy US Forest Service). |
|
Fig. 6. Douglas-fir (Pseudotsuga menziesii) Christmas trees damaged by Swiss needle cast caused by Phaeocryptopus gaeumannii (Wisconsin 1980). (Courtesy US Forest Service). |
|
The pathogen has been introduced into central and eastern North America where Douglas-fir has been planted for Christmas tree production. On the lower branches, rows of black, fuzzy fruit bodies develop on the underside of green and yellow infected needles that turn brown and are cast in late summer. Disease impact has been reduced through applications of fungicides and altered cultural practices such as increasing air circulation at the base of trees to reduce moisture necessary for infection, and avoiding spread of the pathogen on shearing equipment in Christmas tree plantations. Genetic improvement has shown promise and is based on differences in susceptibility to the disease of Douglas-fir trees from different seed sources.
Scleroderris canker. Scleroderris canker and shoot blight of conifers caused by Gremmeniella abietina has been known to be a destructive disease in Europe since the late 1800s but was not recognized in North America until the early 1950s in the Upper Peninsula of Michigan where extensive mortality of young red pine occurred. It was not until 1964 that the causal agent was identified and by 1969 the disease was found in Lower Michigan and Minnesota. A forest tree in Michigan was found to be the source of inoculum and further inadvertent spread of the pathogen on infected nursery stock was halted. The original source of the North American race is unknown; however, there was no evidence that it was native to the Lake States. The most plausible hypothesis is the fungus was introduced on planting stock from Europe when large-scale reforestation from 1929-1944 in the Lake States included imported Scots pine.
Research developed widely applied practices including silvicultural techniques such as avoiding high hazard areas (Fig. 7), planting resistant species, pruning lower branches in plantations, and chemical applications in nurseries. Since 1972 spread of the pathogen on infected nursery stock has been eliminated with the use of sanitation and fungicide treatments. Natural spread from affected plantations continues; however, inoculum levels are reduced as trees have grown out of the susceptible size. Planting in high hazard sites and on sites where the disease is well established has been minimized.
| |

Fig. 7. Red pine (Pinus resinosa) growing in a frost pocket (a high hazard site) killed by Scleroderris canker caused by the North American race of Gremmeniella abietina (1969). (Courtesy US Forest Service). |
|
In 1959 Scleroderris canker and shoot blight was also found in the Adirondack Mountain region of New York in a severely affected plantation of red and Scots pine. This outbreak was later attributed to the European race of G. abietina. The original source of inoculum was unknown but seedlings imported from Germany had been planted in the area as early as 1870. In 1972 the disease was found in the tops of trees 12 to 15 meters tall in New York (Fig. 8), in contrast to in the Lake States where infection is rarely found above 2 meters. The epidemic of the European strain in Eastern forests has subsided, presumably owing to the absence of critical weather conditions now thought to be responsible for the initial outbreak and also because of the various cultural practices employed and quarantines put in place.
| |

Fig. 8. Red pine killed by the European race of Gremmeniella abietina (New York 1976). (Courtesy US Forest Service). |
|
| |

Fig. 9. Current-year shoot of red pine (Pinus resinosa) killed by Sirococcus conigenus. (Courtesy US Forest Service). |
Red pine shoot blights. A shoot blight of red pine caused by Sirococcus conigenus was first reported from Wisconsin in 1959 and became epidemic throughout the Lake States in plantations by the mid-1970s. The pathogen has been reported from several conifer species in Europe, Canada and northern United States where hosts in native stands, nurseries, plantations and landscape plantings can be damaged. The disease is favored by wet spring weather and infection of current year’s shoots (Fig. 9) can kill seedlings and successive outbreaks can result in death of young trees. Conidia are disseminated in spring and early summer with symptoms of drooping, red needles and shoot curling developing in late summer. The fungus is seedborne on several conifer species. In the Lake States disease severity is greatest when red pine is planted under or adjacent to infected overstory red pine so understory trees in multi-aged red pine stands are vulnerable (Fig. 10). The disease has been controlled in nurseries with fungicides.
|
 |
|
Fig. 10. Red pine saplings growing under a red pine canopy killed by Sirococcus conigenus. (Courtesy US Forest Service). |
|
Diplodia pinea is a pathogen of worldwide importance on many pine species. The disease can impact growth rates, deform and kill trees. Stem cankers caused by D. pinea were first reported in North America on red pine in Wisconsin in 1976. The disease is favored by trees under stress and the greatest damage is to trees in nurseries, plantations, windbreaks and plantings of exotic pines (Figs. 11 and 12). Conidia are spread in wet weather and the fungus develops in shoots, bark, cones and litter. Infection of elongating shoots results in brown, stunted or curled current-year shoots of trees of all ages. Outbreaks of Diplodia shoot blight over extensive areas have occurred after hail storms. Inadvertent planting of latently infected seedlings assisted in the widespread distribution of this pathogen. Removal of red pine nursery wind breaks that can harbor the pathogen, the use of fungicides, planting disease-free stock and avoiding planting sites near infected overstory trees have reduced the incidence of this shoot blight.
 |
|
 |
|
Fig. 11. Red pine (Pinus resinosa) nursery seedlings killed by Diplodia pinea (seedlings in background protected by fungicide) (Wisconsin 1981). (Courtesy US Forest Service). |
|
Fig. 12. Red pine in windbreak killed by Diplodia pinea (Wisconsin 1977). (Courtesy US Forest Service). |
Annosum root rot. Heterobasidion annosum, the cause of "death circles" in conifer stands in Europe since at least the 1870s, has been reported from most northern temperate regions and some tropical and subtropical areas of the world (Fig. 13). Until recently, two strains of H. annosum (P and S-types) were recognized in North America. The P-type, currently still regarded as H. annosum, causes major damage in plantations of loblolly, red and slash pine in eastern states and Texas in the USA, and Ontario and Quebec in Canada. The S-type, currently regarded by some experts as an un-named taxon of Heterobasidion, commonly occurs on fir, spruce, Douglas-fir and hemlock from British Columbia to California in western North America. Although H. annosum sensu lato has been known to occur in North America for at least a century, reports of significant damage are more recent. The first reports of southern pine mortality came from Georgia and South Carolina in 1954.
| |

Fig. 13. Fruit bodies of Heterobasidion annosum at the base of a stump. (Courtesy US Forest Service). |
|
Tree mortality in pine plantations occurs within several years after thinning; for example, between 25 and 62 trees per ha of loblolly pine died within 5 to 7 years of the first thinning operation in a southern study. Significant growth loss (height and stem diameter) in loblolly and slash plantations was also first reported in the mid-1960s. The S-type strain is extremely common in western stands with true fir and its importance as a pathogen is expected to increase with changing forest management, e.g., fire control and partial cutting.
Annosum root rot is controllable through consistent use of disease management practices. Control options are greatest for southern pine plantations where hazard rating systems are coupled with stump treatment (e.g., disodium octaborate tetrahydrate [DOT]) and careful timing of thinning operations. The use of mechanized harvesters has been the impetus for recent investigation of liquid application technology in the US; such applications of DOT are commonly used in Scandinavian countries. The range and intensity of the disease in eastern North America is expected to increase with adoption of uneven aged management practices and increased restrictions on the use of prescribed burning.

Fig. 14. Dwarf mistletoe (Arceuthobium pusillum) witches’ brooms on black spruce (Picea mariana). (Courtesy US Forest Service). |
|
Dwarf mistletoes. Dwarf mistletoes (Arceuthobium) are perennial, obligate parasitic seed plants on aerial portions of many Pinaceae species in North America, Newfoundland and Central America and Pinaceae and Cupressaceae in Asia (Fig. 14). All dwarf mistletoes consist of an endophytic rootlike system within the host tissues and a dioecious reproductive aerial shoot system. In western United States dwarf mistletoes have a greater impact on forests than any other pathogen, decreasing growth rates, distorting tree form, reducing wood quality and killing trees (Fig. 15). It has been estimated that over 29 million acres are infested in western forests and Alaska with 164 million cubic feet of wood lost annually. Flowering occurs in spring and seed mature in late summer to fall. Local spread is predominately accomplished by explosive seed dispersal and long distant spread is aided by birds and mammals. The most effective control strategies, where feasible to apply, have been the use of prescribed burning and removal of all symptomatic and asymptomatic trees during harvest.
| |

Fig. 15. Dwarf mistletoe infection center in black spruce. (Courtesy US Forest Service). |
|
Butternut canker. A stem canker was associated for the first time with dying butternut (Juglans cinerea) in southwestern Wisconsin in 1967. The fungus causing the canker Sirococcus clavigignenti-juglandacearum was described as a new species in 1979 and is now thought to be an exotic pathogen with an unknown origin. Butternut of all ages throughout its range in North America is being killed by the disease (Fig. 16). Cankers are elongated sunken areas often with a black center and whitish margin. Coalescing perennial stem cankers eventually girdle and kill affected trees (Fig. 17). Butternut is the only species killed by the disease, however, the fungus has been found on black walnut (J. nigra) and heartnut (J. ailanthifolia var. cordiformis). Seedlings of other hardwood species and grafted plants of other Juglans species are susceptible to varying degrees when inoculated with S. clavigignenti-juglandacearum in greenhouse studies. The devastating impact of the canker has led many states and National Forests to list butternut as species of special concern and many management agencies have established policies to retain the species. In Canada butternut is listed as endangered. Strategic plans for the conservation and restoration of butternut are being developed in the United States and Canada. Butternut trees with putative resistance to the disease have been clonally propagated and established in seed orchards, and silvicultural practices to retain and regenerate butternut in natural stands are being tested.
 |
|
 |
|
|
Fig. 16. Butternut (Juglans cinerea) trees killed by butternut canker (Wisconsin 1975). (Courtesy US Forest Service). |
|
Fig. 17. Coalescing cankers caused by Sirococcus clavigignenti-juglandacearum on the lower stem of an infected butternut. (Courtesy US Forest Service). |
|
Pitch canker. Pitch canker of pines was first described in the mid-1940s in the southeastern USA. Despite the disease name, the causal fungus Fusarium circinatum (teleomorph: Gibberella circinata) infects a variety of vegetative and reproductive plant parts at different stages of maturity, resulting in a variety of symptoms (Fig. 18). The classic symptom is a resin-exuding canker on the trunk, terminal shoots, or large branches of susceptible pines. Another common symptom is dieback that results from cankers forming on shoots flushing in late summer in the upper crown of infected pines (Fig. 19). Between 1945 and 1973 limited disease out-breaks were reported in slash and Virginia pine stands in this region, but the disease was not considered economically important. Epidemics were first reported in 1974 in slash pine in Florida plantations and loblolly pine in seed orchards in North Carolina and Mississippi. By the mid-1980s, over 551,000 acres of slash pine were affected by the disease with an average disease incidence of 14% per stand. Annual volume loss and cull due to the disease on slash pine are perhaps more significant than the annual mortality caused (< 2%).
| |
 |
|
 |
|
| |
Fig. 18. Slash pine (Pinus elliottii) with dieback caused by pitch canker. (Courtesy US Forest Service). |
|
Fig. 19. Slash pine shoot killed by pitch canker. (Courtesy US Forest Service). |
|
In the mid-1980s, significant dieback occurring in Monterrey pine landscape plantings in Santa Cruz County, California, was attributed to the pathogen. This find represented a major jump in the pathogen’s previously known range, i.e., Virginia to southern Florida and west to eastern Texas. The pathogen is now considered a recent introduction to California from the well established F. circinatum lineage in the southeastern USA. Its introduction most likely resulted from shipment and outplanting of infected, but asymptomatic, seedlings that originated in a southeastern US pine nursery where pitch canker was well established. In California, pitch canker’s impact thus far has been most significant in adolescent and mature Monterrey pines in landscape plantings, such as highway rights-of-way and golf courses. Insects play a critical role as wounding agents and vectors in the California situation; whereas, natural and manmade wounding appears most important in new infections in the Southeast. Although the disease is considered endemic in the latter region, the appearance and periodic occurrence of epidemics may be a consequence of intensive forest management practices. In California, the pathogen could likely build-up on other pine hosts and spread through contiguous plantings on forests of these species, leading to future expansion of the pitch canker range in the state. Environmental limitations, however, could limit the disease distribution to coastal areas.

Fig. 20. Crown of northern red oak (Quercus rubra) affected by oak wilt (1959). (Provided by D.W. French to J. Juzwik). |
|
Oak wilt. Although dying oaks with wilting foliage were noted in the late 1800s, the causal fungus (Ceratocystis fagacearum) was not recognized as the cause of oak wilt until 1942. Initially, the fungus was isolated from wilting trees in various counties in the Upper Midwest, e.g., 23 counties in Wisconsin and a few counties in Illinois, Iowa and Minnesota (Fig. 20). Currently the disease occurs in 860 counties of 23 states of the USA. It is not known to occur elsewhere. Although more than 33 species of oak (Quercus spp.) are known to be susceptible to the pathogen, the species that are primarily affected include northern pin, live, bur, northern red, shumard, and Texas red oak. The foliar symptoms vary largely by oak species group (red or Section Lobatae; white or Section Quercus). Leaf bronzing from leaf margins to the midrib and from leaf apex to base is characteristic of red oak species. Red oak species also die within months to one year following infection (Fig. 21). Widely varying symptoms occur on white oak species and death occurs over years.
| |
Fig. 21. Mirror image of sporulation mat produced by the oak wilt fungus, Ceratocystis fagacearum, in the cambial area of a recently wilted northern red oak (Quercus rubra). (Courtesy J. Juzwik). |
|
 |
|
n the 1940s and 1950s, great concern existed about the potential for the pathogen to cause widespread epidemics and massive mortality of oaks because the disease discovery followed in the wake of chestnut blight and Dutch elm disease. Fortunately, the disease has never reached the predicted or potential levels. Sub-regional and local epidemics have occurred in the Upper Midwest and in Texas. Oak wilt is the primary forest disease of concern in Iowa, Minnesota, Illinois, Texas and Wisconsin. Tens to hundreds of thousands of oaks die annually in the Upper Midwest and in Texas. The significance of oak wilt in the urban and peri-urban forests has increased greatly since the late 1970s, while its importance in timberland has either lessened or not changed.
Oak wilt suppression programs involving federal–state cost-share funding have been important in managing the disease in Minnesota and Texas. Overall, the disease range has remained relatively static; however, significant expansions have occurred within several states. Oak wilt is a manageable disease, but requires consistent and long-term control efforts. The potential still exists for the pathogen to be unintentionally introduced and established in other parts of North America with susceptible oak forests.
Bacterial leaf scorch. Bacterial leaf scorch (BLS) of landscape trees is not a new disease; rather, it is a disease with a fairly recently (1987) discovered pathogen, Xylella fastidiosa now considered to be X. fastidiosa subsp. multiplex. It has likely been mis-diagnosed or considered insignificant prior to the pathogen’s recognition. BLS has been found on shade trees in the east from New York southward to Florida and westward to Nebraska and southward to Oklahoma and Texas as well as in California. The significance and impact of BLS on elm, oak and sycamore were first recognized during the latter two decades of the 20th century. A survey in New Jersey found X. fastidiosa present in 39% of 1,372 samples collected statewide. Infected oaks may die within 4 to 6 years of contracting the bacterium (Fig. 22). Mortality and rapid increase in disease incidence were also reported for planted London plane trees in Charlotte, NC. The importance of BLS in urban forests is clearly growing, while its significance in planted and natural forests remains to be determined. Although spread by grafting occurs in at least some species, spread by insect vectors such as spittlebugs, leafhoppers and treehoppers are considered the reason for rapid increases in incidence and spread.
| |

Fig. 22. Crown of southern red oak (Quercus falcata) affected by bacterial leaf scorch. (Courtesy: P. Spaine). |
|
Symptoms of BLS in oak and elm may be confused with those caused by the vascular fungal pathogens, Ceratocystis fagacearum and Ophiostoma novo-ulmi, respectively. Marginal leaf discoloration and necrosis are found on leaves of X. fastidiosa infected elms and oaks, while drab, olive-colored leaves that quickly turn brown are characteristic of infected sycamores. In hosts of all three genera, foliar branch symptoms progress from older to younger leaves. Progressive crown dieback results from infection in elms, oaks and sycamores. Tree death caused by C. fagacearum in red oaks occurs within 1 or 2 years of infection compared to 4 to 6 years in X. fastidiosa infected trees. No management methods are available to stop the spread of the disease. Cultural practices are used to maintain tree vigor in order to extend the life of a BLS affected tree. Systemic treatment with antibiotics results in symptom remission, but does not eradicate the pathogen from infected trees. Trees with extensive leaf scorch and dieback are removed.
| |

Fig. 23. Leaf scorch of elm (Ulmus americana) caused by Xylella fastidiosa. Photo by J.L. Sherald, from the APS digital image collection, Diseases of Woody Ornamentals and Trees). |
|
Concluding Remarks
The underlying factor in most of the disease examples presented above is that the problems arose either through the inadvertent movement of pathogens, the host or both or previously minor problems became prominent because of changes in management systems employed and/or local environmental conditions that exacerbated previously minor problems. In any case, the pathogens became more invasive and damaging than in the past and forest pathologists were called on to identify the causes and develop solutions to these new problems. This review of selected disease examples from the last century support the assertion that forest pathologists have succeeded to varying degrees in developing management strategies, however, in most cases our forest ecosystems have been forever changed by major diseases. Looking forward in the 21st century, increasing global trade, continued forest fragmentation, changing public needs, changing values placed on forest and landscape trees, and climate change will certainly provide new challenges for the next generation of forest pathologists.
Resources for Further Reading
Anderson, R. L. 2003. Changing forests and forest management policy in relation to dealing with forest diseases. Phytopathology 93:1041-1043.
Appel, D. N. 2001. The basics of oak wilt biology and factors influencing disease incidence and severity. Pages 71-81 in: Shade Tree Wilt Disease. C. L. Ash, ed. American Phytopathological Society, St. Paul, MN.
Chastagner, G. A., and Benson, D. M. 2000. The Christmas tree: Traditions, production, and diseases. Online. Plant Health Progress doi:10.1094/PHP-2000-1013-01-RV.
Cordell, C. E., Anderson, R. L., Hoffard, W. H., Landis, T. D., Smith, R. S., Jr., and Toko, H. V. 1989. Forest Nursery Pests. Agric. Handb. No. 680. US Forest Serv., USDA, Washington, DC.
Dwinell, L. D., Barrows-Broaddus, J. B., and Kuhlman, E. G. 1985. Pitch canker: A disease complex of southern pines. Plant Dis. 69:270-276.
Gordon, T. R., Storer, A. J., and Wood, D. L. 2001. The pitch canker epidemic in California. Plant Dis. 85:1128-1139.
Gould, A. B., and Lashomb, J. H. 2005. Bacterial leaf scorch of shade trees. APSnet Features, November 2005. American Phytopathological Society, St. Paul, MN.
Hansen, E. M., and Lewis, K. J., eds. 1997. Compendium of Conifer Diseases. American Phytopathological Society, St. Paul, MN.
Hansen, E. M., Stone, J. K., Capitano, B. R., Rosso, P., Sutton, W., Winton, L., Kanaskie, A., and McWilliams, M. G. 2000. Incidence and impact of Swiss needle cast in forest plantations of Douglas-fir in coastal Oregon. Plant Dis. 84:773-778.
Hawksworth, F. G., and Wiens, D. 1996. Dwarf mistletoes: Biology, Pathology, and Systematics. Agric. Handb. No. 709. US Forest Serv., USDA, Washington, DC.
Kinloch, B. B., Jr. 2003. White pine blister rust in North America: Past and prognosis. Phytopathology 93:1044-1047.
Manion, P. D., ed. 1984. Scleroderris Canker of Conifers. Springer-Verlag, The Netherlands.
Manion, P. D. 2003. Evolution of concepts in forest pathology. Phytopathology 93:1052-1055.
MacDonald, W. L. 2003. Dominating North American forest pathology issues of the 20th century. Phytopathology 93:1039-1040.
McCullough, D. G., Katovich, S. A., Ostry, M. E., and Cummings-Carlson, J. 1998. Christmas Tree Pest Manual. Ext. Bull. E-2676, Michigan State Univ., Northeastern Area State and Private Forestry, North Central Forest Exp. Stn., US Forest Serv. USDA, Washington, DC.
Michler, C. M., Pijut, P. M., Jacobs, D. F., Meilan, R., Woeste, K. E., and Ostry, M. E. 2006. Improving disease resistance of butternut (Juglans cinerea), a threatened fine hardwood: a case for single-tree selection through genetic improvement and deployment. Tree Physiol. 26:121-128.
O’Brien, J. T. 1973. Sirococcus shoot blight of red pine. Plant Dis. Rep. 57:246-247.
Schmidt, R. A. 2003. Fusiform rust of southern pines: a major success for forest disease management. Phytopathology 93:1048-1051.
Sinclair, W. A., and Lyon, H. H. 2005. Diseases of trees and shrubs. Cornell Univ., Ithaca, NY.
Tainter, F. H. 2003. Perspectives and challenges from the 20th century. Phytopathology 93:1056-1061.
Walla, J. A., Jacobi, W. R., and Schmidt, R. A. 2003. Forest pathology for the last century: An overview of the symposium. Phytopathology 93:1037-1038.
Woodward, S., Stenlid, J., Karjalainen, R., and Hutterman, A. 1998. Heterobasidion annosum: Biology, Ecology, Impact and Control. CAB Intn'l., Wallingford, UK.
