
 Figure 1. An American chestnut stem with a chestnut blight canker. (Click image for larger view and more information). Figure 1. An American chestnut stem with a chestnut blight canker. (Click image for larger view and more information). |
|
Chestnut blight, or chestnut bark disease, is caused by an introduced fungus,
Cryphonectria parasitica (Murrill) Barr, (formerly
Endothia parasitica [Murrill] Anderson & Anderson). The fungus enters wounds, grows in and under the bark (Fig. 1), and eventually kills the cambium all the way around the twig, branch, or trunk (33). Sprouts develop from a burl-like tissue at the base of the tree called the ‘root collar,’ which contains dormant embryos (39). Sprouts grow, become wounded and infected, and die, and the process starts all over again.Cankers were first reported in the United States in 1904 on American chestnut trees (Castanea dentata [Marshall] Borkhausen) (Fig. 2) in New York City (32). None of the control attempts (chemical treatments, clearing and burning chestnut trees around infection sites) were successful (47). By 1926 the fungus was reported throughout the native range of American chestnut (Fig. 3), and a major forest tree had been reduced to a multiple-stemmed shrub (17). In 1912 the Plant Quarantine Act was passed to reduce the chances of such a catastrophe happening again (49).

Figure 2. An American chestnut tree (Castanea dentata [Marshall] Borkhausen) growing in Scotland, Connecticut in 1905. The tree was 83 feet tall, 27 inches in diameter, and 103 years old. (Click image for larger view). | |
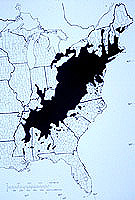
Figure 3. The natural range of American chestnut as presented by Saucier in 1973. (Click image for larger view). |
The fungus was already wide spread in the north-eastern U.S. by 1904, but there were no reports of it south of Virginia (34). Metcalf and Collins suggested that Japanese chestnut trees (C. crenata Siebold and Zuccarini), which were first imported in 1876 (40), were the source of the pathogen (Fig. 4). A large number of grafted and seedling Japanese chestnuts were imported by 1900 (40), and it was clear that diseased nursery stock was the most important factor in the spread of chestnut blight to distant points. By 1900, many of the major mail-order nurseries offered Japanese (and European) chestnut trees for sale throughout the country (5).
|

Figure 4. A Japanese chestnut tree (Castanea crenata Siebold and Zuccarini) planted in Old Lyme, Connecticut in 1876 and photographed in 2000. (Click image for larger view). | |
In 1913, David Fairchild of the U.S.D.A. asked plant explorer Frank Meyer to look for the disease in Asia. Meyer reported in early June that he had found it in China, and sent samples (16). Shear and Stevens grew cultures from Meyer's samples, and in July they inoculated the Chinese fungus into American chestnut trees near Washington, D.C.; yet another introduction of the fungus into the forest (44). Rapid death of the sprouts confirmed that this similar-appearing fungus caused chestnut blight. Meyer found chestnut blight disease in Japan in 1915 (45), and we now know that Japanese trees and some Chinese trees have good resistance to the fungus, and although they may be infected they are rarely killed.
The blight fungus moves from tree to tree as spores on the feet, fur, and feathers of the many animals and insects that walk across the cankers (42,43,48). Sexual spores (ascospores) are shot into the air after rain storms in the fall and are another source of infection (9,41). The disease is now throughout the native range of American chestnut, and has moved into some of the places where trees were planted outside the range.
There has been little chance for resistance to evolve in American chestnut, since the sprouts that come up after trees are killed by chestnut blight disease are often killed before they become sexually mature. Since two flowering chestnut trees are needed for seed formation (they must be cross-pollinated), sexual reproduction has been drastically reduced. In the full sun of a clear-cut the chestnut blight cycle of sprouting-infection-death-sprouting takes about ten years, and in the understory of a forest the cycle may take 40 years (6,23). Griffin found that many chestnuts in two sites in Virginia did not sprout after dying from chestnut blight. Both of these sites had much higher nitrogen levels than sites with good survival, and the site with poorest survival had very high levels of calcium in the soil (24). In spite of this, in some places American chestnut root sprouts are still a major component of sub-canopy and shrub biomass (1,31,38,46).
A Parasite Imported to Control Chestnut Blight

Figure 5. Heavily callused chestnut blight cankers on American chestnut trees after treatment with hypovirulent strains of
Cryphonectria parasitica. (Click image for larger view).

Figure 6. American chestnut trees with multiple hypovirulent cankers. The last treatments with the biocontrol strains in this Connecticut orchard was 19 years previous to this photograph. (Click image for larger view). | |
An imported parasite has given us a partial solution to the control of chestnut blight disease. Jene Grente reported in 1965 that ‘hypovirulent’ strains of the blight fungus from Italy lacked the ability to kill chestnut trees, and that inoculation of these strains into existing (lethal) cankers resulted in canker remission (18). Grente published several papers describing these strains with less than normal virulence, and began a program of active intervention when blight was found in France (19,20,21). Treatment of new cankers as they formed resulted in a successful ‘biological therapy’ of the disease. After four or five years of therapy, hypovirulent strains began to spread through the chestnut orchards of France, the trees began ‘healing’ over the blight cankers with bark-callus tissue, and a biological control was established (Fig. 5) (22).
We imported some of Grente’s hypovirulent strains in 1972, and were sent samples from swollen cankers from Michigan, Tennessee, Virginia, and West Virginia which yielded strains of the blight fungus that were also less able to kill chestnut trees. We found that Grente's strains, and the U.S. hypovirulent strains, contained dsRNA, and that blight strains with dsRNA could pass hypovirulence to lethal, American strains on American chestnut trees (14). After getting permission from Plant Quarantine, we used hypovirulent strains to treat cankers in orchards and forests, putting pieces of the hypovirulent strains growing on an agar medium into holes in the bark around killing cankers, and found that single introductions of hypovirulent strains into an area did little more than stop the expansion of the treated cankers (8). By treating every canker that we could reach for at least four years, on a large group of trees, we have established biological control of chestnut blight disease in American chestnuts in Connecticut (3,6). It works much better in an orchard situation, where the trees are close together, and the various carriers don’t have far to walk or fly to the next tree, than it does in a forest where the sprouts are often suppressed by shade and competition for nutrients, and are mixed in a collection of oaks, maples, and shrubs. However, even in forest plots this biological control can keep trees alive and flowering. The trunks are severely disfigured by the swollen cankers, and they would probably not be very useful for timber (Fig. 6) (6,25,30).
The transmission of hypovirulence from strain to strain of the fungus is restricted by a genetic system of vegetative incompatibility (Fig. 7) (2). Preliminary genetic studies by Huber were extended by Cortesi and Milgroom who estimate that there are six loci, each with two alleles in a system of heterogenic incompatibility which keep the strains of the fungus from fusing and passing hypovirulence (13,27). Milgroom has used this system and molecular markers to examine strains from populations of
C. parasitica throughout the world (35). Similar studies in Europe have extended our knowledge of the populations of this fungus world wide (10).
 | |
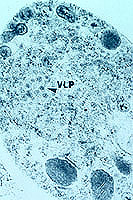 | |
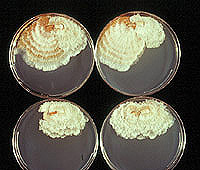
Figure 7. Pairs of virulent and hypovirulent strains of
Cryphonectria parasitica on Difco Potato Dextrose Agar. (Click image for larger view and more information). | |
Figure 8. Virus-like particles in a
Cryphonectria parasitica cell. (Click image for larger view). | |
Recent work with the dsRNA found in hypovirulent strains has confirmed that this is the genetic material of a fungal virus (Fig. 8). Hillman and his co-workers have named it as a new genus (26). There is now a great deal of work being done with the viral genome and the effects of the genes on the fungus, and Nuss has recently reviewed this work (37). Several viruses have now been identified, and a mitochondrial DNA mutation which causes hypovirulence is also being studied (15,36). The viral genome has been inserted into the fungal genome, producing a stable recombinant which produces viral particles into the cytoplasm (12). This construct allows production of ascospores with the viral genome (7).
Breeding Programs
|
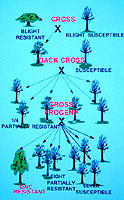
Figure 9. Crossing plan used by The Connecticut Agricultural Experiment Station and The American Chestnut Foundation for producing chestnut trees with American form and Asian blight resistance. This diagram is based on the assumption that there are two genes in Asian chestnuts responsible for blight resistance. (Click image for larger view). | |
Our breeding plan was first based simply on making hybrids of resistant Asian trees with susceptible American trees and testing the hybrids for resistance to chestnut blight (4,29). When it became clear that at least two genes were responsible for this resistance, we began a back-cross breeding system based on the plan of Charles Burnham (11). Resistant Asian trees are crossed with susceptible American trees, and the partially resistant hybrids are crossed to American trees again. One out of four of the progeny from these crosses have one copy of both resistance genes (giving them partial resistance), and these are crossed again to American. This repeated back-crossing increases the percentage of American genes in the hybrids, and selecting for partial resistance insures passage of the resistance genes. A final cross of two trees with partial resistance should result in one of sixteen trees having two copies of both resistance genes, which will make them fully resistant to the chestnut blight fungus (Fig. 9).
The breeding work has been greatly helped by a genetic map prepared by Kubisiak (28). He has RAPD markers for the chromosomal regions associated with resistance to chestnut blight, which will make selection much easier. Trees of two kinds are being chosen: for timber and for nut production, both with resistance to chestnut blight.

Figure 10. Pure stand of American chestnut trees in Connecticut in 1905. After clear-cutting (90 years prior to this photograph), the chestnuts out-competed all other woody species and grew back as a monoculture. (Click image for larger veiw).
| |
At the very least, we will be able to maintain American trees as fruiting populations. Then if we plant our new resistant hybrids out into these plots, they will cross with native trees, incorporating the enormous genetic diversity that still exists in the forest. The first generation offspring will be intermediate in resistance, but in subsequent generations trees with full resistance will be produced. These will be well adapted to all the regions of the country where such plantings have been made, and should compete well with the additional help of biological control by hypovirulence. The hope is that chestnut trees may again become a usable timber resource in the forests of the world
(Fig. 10).
Additional Resources
The Connecticut Agricultural Experiment Station
Northern Nut Grower's Association, Inc.
The American Chestnut Foundation
American Chestnut Cooperators' Foundation
References
1. Adams, S. M. and Stephenson, S. L. 1983. A description of the vegetation on the south slopes of Peters Mountain, southwestern Virginia. Bulletin of the Torrey Botanical Club 110:18-23.
2. Anagnostakis, S. L. 1977. Vegetative incompatibility in
Endothia parasitica. Experimental Mycology 1:306-316.
3. Anagnostakis, S. L. 1990. Improved chestnut tree condition maintained in two Connecticut plots after treatments with hypovirulent strains of the chestnut blight fungus. Forest Science 36:113-124.
4. Anagnostakis, S. L. 1992. Measuring resistance of chestnut trees to chestnut blight. Canadian Journal of Forest Research 22:568-571.
5. Anagnostakis, S. L. 1995. The Pathogens and Pests of Chestnuts. Pages 125-145 in: Advances in Botanical Research, vol. 21, J. H. Andrews and I Tommerup, eds. Academic Press, New York.
6. Anagnostakis, S. L. 2001. American chestnut sprout survival with biological control of the chestnut-blight fungus population. Forest Ecology and Management (in press).
7. Anagnostakis, S. L., Chen, B., Geletka, L. M., and Nuss, D. L. 1998. Hypovirus transmission to ascospore progeny by field-released transgenic hypovirulent strains of
Cryphonectria parasitica. Phytopathology 88:598-604.
8. Anagnostakis, S. L. and Jaynes, R. A. 1973. Chestnut blight control: use of hypovirulent cultures. Plant Disease Reporter 57:225-226.
9. Anderson, P. J. 1914. The morphology and life history of the chestnut blight fungus. Pennsylvania Chestnut Tree Blight Commission bulletin 7, Harrisburg PA.
10. Bissegger, M., Rigling, D., and Heiniger, U. 1997. Population structure and disease development of
Cryphonectria parasitica in European chestnut forests in the presence of natural hypovirulence. Phytopathology 87:50-59.
11. Burnham, C. R. 1988. The restoration of the American chestnut. American Scientist 76:478-487.
12. Choi, G. H. and Nuss, D. L. 1992. Hypovirulence of chestnut blight fungus conferred by an infectious viral cDNA. Science 257:800-803.
13. Cortesi, P. and Milgroom, M. G. Genetics of vegetative incompatibility in
Cryphonectria parasitica. Applied and Environmental Microbiology 64:2988-2994.
14. Day, P. R., Dodds, J. A., Elliston, J. E., Jaynes, R. A., and Anagnostakis, S. L. 1977. Double-stranded RNA in Endothia parasitica. Phytopathology 67:1393-1396.
15. Enebak, S. A., MacDonald, W. L., and Hillman, B. I. 1994. Effect of dsRNA associated with isolates of
Cryphonectria parasitica from the central Appalachians and their relatedness to other dsRNAs from North America and Europe. Phytopathology 84:528-534.
16. Fairchild, D. 1913. The discovery of the chestnut bark disease in China. Science 38:297-299.
17. Gravatt, G. F. and Marshall, R. P. 1926. Chestnut blight in the Southern Appalachians. U.S.D.A. circular 370, Washington, D.C.
18. Grente, J. 1965. Les formes Hypovirulentes d'Endothia parasitica et les espoirs de lutte contre le chancre du châtaignier. Académie d’Agriculture de France, Extrait du Proces-verbal de la Séance. 51:1033-1037.
19. Grente, J. 1975. La lutte biologique contre le chancre du châtaignier par “Hypovirulence contagieuse.” Annales de Phytopathologie 7:216-218.
20. Grente, J. and Sauret, S. 1969a. L’hypovirulence exclusive, phénomene original en pathologie végétale. Comptes Rendus Hebdomadaire des Séances de l’Académie d’Agriculture de France. Série D 286:2347-2350.
21. Grente, J. and Sauret, S. 1969b. L’”hypovirulence exclusive” est-elle contrôlée par des déterminants cytoplasmiques? Comptes Rendus Hebdomadaire des Séances de l’Académie d’Agriculture de France. Série D 286:3173-3176.
22. Grente, J. and Berthelay-Sauret, S. 1978. Biological control of chestnut blight in France. IN: “Proceedings of the American Chestnut Symposium.” W. L. MacDonald, F. C. Cech, J. Luchock, and C. Smith, eds. pp. 30-34. West Virginia University, Morgantown.
23. Griffin, G. J. 1989. Incidence of chestnut blight and survival of American chestnut in forest clearcut and neighboring understory sites. Plant Disease 73:123-127.
24. Griffin, G. J. 1992. American chestnut survival in understory mesic sites following the chestnut blight pandemic. Canadian Journal of Botany 70:1950-1956.
25. Heiniger, U. and Rigling, D. 1994. Biological control of chestnut blight in Europe. Annual Review of Phytopathology 32:581-599.
26. Hillman, B. I., Fulbright, D. W., Nuss, D. L., Van Alfen, N. K. 1994. Hypoviridae. IN: “Virus Taxonomy: Sixth Report of the International Committee for the Taxonomy of Viruses” F. A. Murphy, C. M. Fauquet, D. H. L. Bishop, S. A. Ghabrial, A. W. Jarvis, G. P. Martelli, M. P. Mayo, and M. D. Summers, eds. Springer-Verlag, Wein, New York, 580 pp.
27. Huber, D. H. and Fulbright, D. W. 1994. Preliminary investigations on the effect of individual vic genes upon the transmission of dsRNA in Cryphonectria parasitica. pp 15-19 IN: “Proceedings of the International Chestnut Conference” M. L. Double and W. L. MacDonald, eds., West Virginia University Press, Morgantown.
28. Kubisiak, T. L., F. V. Hebard, C. D. Nelson, J. Zhang, R. Bernatzky, H. Huang, S. L. Anagnostakis, and R. L. Doudrick. 1997. Mapping resistance to blight in an interspecific cross in the genus Castanea using morphological, isozyme, RFLP, and RAPD markers. Phytopathology 87:751-759.
29. Lee, J. K., Tattar, T. A., Berman, P. M., and Mount, M. S. 1992. A rapid method for testing the virulence of Cryphonectria parasitica using excised bark and wood of American chestnut. Phytopathology 82:1454-1456.
30. MacDonald, W. L. and Fulbright, D. W. 1991. Biological control of chestnut blight: use and limitations of transmissible hypovirulence. Plant disease 75:656-661.
31. McCormick, J. E. and Platt, R. B. 1980. Recovery of an Appalachian forest following the chestnut blight. American Midland Naturalist 104:264-273.
32. Merkel, H. W. 1905. A deadly fungus on the American chestnut. N.Y. Zoological Society, 10th Annual Report, p 97-103.
33. Metcalf, H. 1912. The chestnut bark disease. p. 363-372 IN: “Yearbook of the Department of Agriculture for 1912,” Washington, D.C.
34. Metcalf, H. and Collins, J. F. 1909. The present status of the chestnut bark disease. U.S.D.A. Bulletin 141 part 5, Washington, D.C., p 45-53.
35. Milgroom, M. G. 1995. Population biology of the chestnut blight fungus, Cryphonectria parasitica. Canadian Journal of Botany 73(s1):s311-S319.
36. Monteiro-Vitorello, C. B., Bell, J. A., Fulbright, D. W., and Bertrand, H. 1995. A cytoplasmically transmissible hypovirulence phenotype associated with mitochondrial DNA mutations in the chestnut blight fungus Cryphonectria parasitica. Proceedings of the National Academy of Science 92:5935-5939.
37. Nuss, D. L. 2000. Hypovirulence and chestnut blight: from the field to the laboratory and back. pp149-170, IN: “Fungal Pathology,” J. W. Kronstad, ed., Kluwer Academic Publishers, Netherlands.
38. Paillet, F. L. 1982. Ecological significance of American chestnut in the Holocene forests of Connecticut. Bulletin of the Torrey Botanical Club 109:457-473.
39. Paillet, F. L. 1993. Growth form and life histories of American chestnut and Allegheny and Ozark chinquapin at various North American sites. Bulletin of the Torrey Botanical Club 120:257-268.
40. Powell, G. H. 1898. The European and Japanese chestnuts in the Eastern United States. Delaware College Agricultural Experiment Station bulletin 42, Newark, DE, 16 pp.
41. Rankin, W. H. 1912. The chestnut tree canker disease. Phytopathology 2:99.
42. Russin, J. S., Shain, L., and Nordin, G. L. 1984. Insects as carriers of virulent and cytoplasmic hypovirulent isolates of the chestnut blight fungus. Journal of Economic. Entomology 77:838-846.
43. Sharf, S. S. and DePalma, N. K. 1981. Birds and mammals as vectors of the chestnut blight fungus (Endothia parasitica). Canadian Journal of Zoology 59:1647-1650.
44. Shear, C. L. and Stevens, N. E. 1913. The chestnut-blight parasite (Endothia parasitica) from China. Science 38:295-297.
45. Shear, C. L. and Stevens, N. E. 1916. The discovery of the chestnut blight parasite (Endothia parasitica) and other chestnut fungi in Japan. Science 43:173-176.
46. Stephenson, S. L., Adams, H. S., and Lipford, M.. 1991. The present distribution of chestnut in the upland forest communities of Virginia. Bulletin of the Torrey Botanical Club 118:24-32.
47. Stoddard, E. M. and Moss, A. E. 1913. The chestnut bark disease. Bulletin 178, The Connecticut Agricultural Experiment Station, New Haven.
48. Studhalter, R. A. and Ruggles, A. G. 1915. Insects as carriers of the chestnut blight fungus. Bulletin 12. Pennsylvania Department of Forestry.
49. Waterworth, H. E. and White, G. A. 1982. Plant introductions and quarantine: the need for both. Plant Disease 66:87-90.
