Peer-Reviewed by Plant Health Progress
Accepted for publication 20 December 2002. © 2003 Plant Management Network.
J. W. Buck, Assistant Professor, Department of Plant Pathology, The University of Georgia, Georgia Experiment Station, Griffin 30223;
R. R. Walcott, Assistant Professor, Department of Plant Pathology, The University of Georgia, Athens 30606;
L. R. Beuchat, Center for Food Safety, The University of Georgia, Griffin 30223
Corresponding author: J. W. Buck. jbuck@griffin.peachnet.edu
Buck, J. W., Walcott, R. R., Beuchat, L. R. 2003. Recent trends in microbiological safety of fruits and vegetables. Online. Plant Health Progress doi:10.1094/PHP-2003-0121-01-RV.
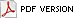
The number of documented outbreaks of human infections associated with the consumption of raw fruits, vegetables, and unpasteurized fruit juices has increased in recent years. According to the Centers for Disease Control and Prevention, in the U.S. the number of reported produce-related outbreaks per year doubled between the period 1973-1987 and 1988-1992 (2,24). During both time periods, the etiologic agent was unknown in more than 50% of outbreaks. Outbreaks with identified etiology were predominantly of bacterial origin, primarily Salmonella. More recently, salmonellosis has been linked to tomatoes, seed sprouts, cantaloupe, mamey, apple juice, and orange juice (6). Escherichia coli O157:H7 infection has been associated with lettuce, sprouts, and apple juice, and enterotoxigenic E. coli has been linked to carrots. Documented associations of shigellosis with lettuce, scallions, and parsley; cholera with strawberries; parasitic diseases with raspberries, basil, and apple cider; hepatitis A virus with lettuce, raspberries, and frozen strawberries; and Norwalk/Norwalk-like virus with melon, salad, and celery have been made. Among the greatest concerns with human pathogens on fresh fruits and vegetables are enteric pathogens (e.g., E. coli O157:H7 and Salmonella) that have the potential for growth prior to consumption or have a low infectious dose. Bacterial pathogens have been isolated from a wide variety of fresh produce (Table 1).
Table 1. Examples of fresh produce and juice from which bacterial pathogens have been isolated.
| Pathogen |
Product |
| Aeromonas |
alfalfa sprouts, asparagus, broccoli, cauliflower, celery, lettuce, pepper, spinach |
| Bacillus cereus |
alfalfa sprouts, cress sprouts, cucumbers, mustard sprouts, soybean sprouts |
| Campylobacter jejuni |
green onions, lettuce, mushroom, potato, parsley, pepper, spinach |
| Clostridium botulinum |
cabbage, mushrooms, pepper |
| E. coli O157:H7 |
alfalfa sprouts, apple juice, cabbage, celery, cilantro, coriander, cress sprouts, lettuce |
| Listeria monocytogenes |
bean sprouts, cabbage, chicory, cucumber, eggplant, lettuce, mushrooms, potatoes, radish, salad vegetables, tomato |
| Salmonella |
alfalfa sprouts, artichokes, beet leaves, celery, cabbage, cantaloupe, cauliflower, chili, cilantro, eggplant, endive, fennel, green onions, lettuce, mungbean sprouts, mustard cress, orange juice, parsley, pepper, salad greens, spinach, strawberries, tomato, watermelon |
| Shigella |
celery, cantaloupe, lettuce, parsley, scallions |
| Staphylococcus |
alfalfa sprouts, carrot, lettuce, onions sprouts, parsley, radish |
| Vibrio cholerae |
cabbage, coconut milk, lettuce |
Adapted from Beuchat (3,6), NACMCF (26,27) and Nguyen-the and Carlin (28)
The potential for widespread outbreaks of human infection caused by consumption of raw produce was dramatically realized during the summer of 1996 in Japan. More than 6000 cases of E. coli O157:H7 infection were reported (15). The largest outbreak resulted in four deaths and affected more than 4000 school children in and around Sakai City. Raw radish sprouts that had been prepared in central kitchens appear to have transmitted the pathogen, although the mechanism of sprout contamination was not determined. In the U.S. between 1995 and 1998, there were nine outbreaks of foodborne illness caused by Salmonella or E. coli O157:H7 due to consumption of fresh vegetable sprouts (27). These outbreaks involved more than 1234 cases in Missouri, Michigan, California, Washington, Arizona, and Nevada, and in most cases, alfalfa or clover seed were implicated as the initial inoculum source. Subsequent outbreaks in Wisconsin have been reported (31). Sprout-related disease outbreaks have also been reported in Japan, the United Kingdom, Finland, Denmark, Sweden, and Canada and have involved alfalfa, cress, radish, and mungbean sprouts (33,42,44).
The increase in food-related outbreaks prompted the U.S. Food and Drug Administration in 1995 to request the National Advisory Committee on Microbiological Criteria for Food (NACMCF) to investigate and characterize the association between cases of foodborne illness and fresh produce and to provide recommendations to reduce the risk of foodborne outbreaks (26). In 1997, the same committee was asked to review the literature on sprout-related disease outbreaks, identify the microorganisms and production practices of greatest public health concern, prioritize research needs, and recommend intervention and prevention strategies (27).
Why Have Produce-Related Human Infections Increased?
Several reasons for the increase in produce-related human infections have been proposed. These include changes in dietary habits, including a higher per capita consumption of fresh or minimally processed fruits and vegetables, and the increased use of salad bars and meals eaten outside the home (1). Yearly consumption of fresh fruits and vegetables in the U.S. has increased by almost 20 pounds per person from 1988 to 1996. This has been attributed to both consumer desire to maintain a healthier diet and the year-round importation of high-quality produce into the U.S. In addition, changes in production and processing methods, sources of produce, and the emergence of pathogens not previously associated with raw produce have enhanced the potential for foodborne illness outbreaks associated with raw fruits and vegetables (17). The end result of these changes is an increased exposure of the general public to fruits and vegetables, which has exacerbated potential problems with contamination by human pathogens.
Sources of Contamination
Determining the exact source of an outbreak is important when devising strategies and interventions to minimize risks of future outbreaks. However, identifying primary inoculum sources for contamination of fresh produce can be tremendously difficult. For example, only two of 27 outbreak investigations described in the NACMCF report on fresh produce clearly identified a point of contamination (26). Unlike other commodities such as beef and chicken that are rigorously inspected, methods to detect pathogens on fresh produce are less advanced, and the sporadic nature of most contamination further limits the effectiveness of testing. Bacterial pathogens may contaminate fruits and vegetables at any point throughout the production system. Potential pre-harvest sources of contamination include soil, feces, irrigation water, water used to apply fungicides and insecticides, dust, insects, inadequately composted manure, wild and domestic animals, and human handling (3). In the production of seeds intended for sprout production, the practice of animal grazing to initiate flowering of alfalfa may result in the introduction of enteric bacteria in feces. Similar consequences may result from allowing wild animals access to seed fields. Non-composted or improperly composted manure can contaminate fruits and vegetables through uses such as a fertilizer or soil amendment, or in irrigation water. Salmonella, E. coli O157:H7, and Listeria monocytogenes can be found in animal feces. Transmission of E. coli O157:H7 from manure-contaminated soil and irrigation water to lettuce plants, and its migration throughout the plant were recently reported (41,46). Evidence of an association of salmonellae with stems and leaves of tomato plants grown hydroponically in inoculated solution has been presented (16). To limit the introduction of pathogenic bacterial through irrigation, the origin and distribution of irrigation water, as well as the history of the land, should be known. Irrigation wells should be well-maintained, and all irrigation sources should be monitored for human pathogens. Manure used as fertilizer should be treated to eliminate pathogenic microorganisms (e.g., composting or aging) and animals (domestic or otherwise) should be excluded from produce and sprout seed production fields. A maximum amount of time also should be scheduled between the final manure application and harvest.
Post-harvest sources of contamination include feces, human handling, harvesting equipment, transport containers, wild and domestic animals, insects, dust, rinse water, ice, transport vehicles, and processing equipment (9). High levels of worker hygiene should be enforced, and human waste management at production sites should follow local laws. A list of FDA good agricultural practices can be found at the U. S. Food and Drug Administration, Center for Food Safety and Applied Nutrition website (45) and should be stringently followed to reduce the risk of pre-harvest contamination of produce.
Seed as an Inoculum Source

Fig. 1. Bean sprouts ready for human consumption. |
|
While the role of seed as a primary inoculum source in plant disease outbreaks is well-established, the significance of seedborne inoculum for human pathogens has been only recently recognized. In many of these cases, vegetable seed sprouts have been implicated as the initial inoculum source. Presently, seed are considered the most significant source of inoculum for foodborne illnesses associated with sprout consumption (27,42) (Fig. 1). Investigation of outbreaks has provided support for this conclusion. For example, between March and June of 1995 there was a marked increase in the number of cases caused by Salmonella Stanley in Michigan, Arizona and Finland (21). For the initial 22 cases, alfalfa sprouts were implicated as the inoculum source, and the bacterial strains isolated from patients in Finland and the U.S. had similar antibiotic resistance and DNA fingerprint profiles (21). However, the pathogens were not detected in the sprouts or samples of the original seedlots. Further investigation indicated that the outbreaks were associated with sprouts from at least nine different producers who obtained their seed from one U.S. distributor. The seed was purchased from a Dutch shipping company who also supplied seed used to produce the sprouts consumed by people in Finland who also became ill. Eventually, 272 cases in 17 U.S. states were associated with this outbreak; however, due to the common practice of blending seedlots, the original source of contaminated seed could not be determined (21). Outbreaks involving other Salmonella enterica serovars (Salmonella Havana, Salmonella Mbandaka, Salmonella Infantis, Salmonella Anatum, Salmonella Newport, Salmonella Montevideo, Salmonella Meleagridis, and Salmonella Senftenberg) shared similar characteristics, including either a common source or identical DNA fingerprints (27,42).
Ecological Factors Influencing Human Pathogens on Food
Little is known about microbial ecosystems on the surface of raw fruits and vegetables. Some produce, such as fully ripe tomatoes, are in a pH range (3.9 to 4.5) that prevents or retards growth of enteric pathogens such as Shigella and E. coli O157:H7. The pH of many vegetables, melons, and soft fruits is 4.6 or higher, which is suitable for the growth of pathogenic bacteria. The growth and survival of human pathogens could be affected by the presence of post-harvest pathogens such as Botrytis cinerea or Penicillium spp. Growth of post-harvest fungi in subsurface tissues can alter the pH of plant tissues, allowing the growth of pathogenic bacteria. Populations of L. monocytogenes inoculated into decayed apple tissue increased on fruit infected by Glomerella cingulata but not by Penicillium expansum (11). This difference was attributed, in part, to the increase in pH of the infected tissues from 4.7 to 7.0 as a result of infection by G. cingulata compared to a decrease in pH from 4.7 to 3.7 as a result of infection by for P. expansum (11). Similar results were obtained with E. coli O157:H7 when co-inoculated with G. cingulata or P. expansum on apple (35). Damaged tissue or lesions on plant surfaces produced by post-harvest spoilage fungi or bacteria could also affect microbial growth due to the presence of nutrients or numerous phytoalexins and other antimicrobial compounds in exudates. In a study of more than 500 samples, each of healthy or soft rotted vegetables and fresh fruits, the incidence of suspected Salmonella spp. on produce affected by bacterial soft rot (e.g., Erwinia carotovora, Pseudomonas fluorescens, and Pseudomonas viridiflava) was twice that of healthy samples (50). More information is needed on the interactions between plant pathogenic fungi or bacteria and the survival and growth of human pathogenic bacteria on produce.
Interactions between human pathogens and the resident, non-pathogenic microflora have been studied in dairy and meat products (28), but little is known about these interactions on fruit and vegetable surfaces (Fig. 2). Large differences in surface morphology and metabolic functions of different plant organs (e.g., fruits, flowers, leaves, roots) provide a wide range of diverse ecological niches that could be selective for specific species or communities of microorganisms. Microbial growth on raw fruits and vegetables can result in the formation of biofilms by spoilage and non-spoilage microorganisms. These biofilms can provide a protective environment for pathogens and reduce the effectiveness of sanitizers and other inhibitory agents. For example, L. monocytogenes, in a multi-species biofilm with Pseudomonas fragi and Staphylococcus xylosus, has been reported to be essentially unaffected by treatment with 500 ppm free chlorine (29). Biofilms have been observed on numerous leaf surfaces, including spinach, lettuce, Chinese cabbage, celery, basil, parsley, and endive (25). No information is available on the behavior of pathogenic bacteria in biofilms formed by the microflora associated with raw fruits and vegetables. The species composition of biofilms on various container and equipment surfaces used in the produce industry would also be predicted to differ greatly, depending on the type of produce being harvested or processed. These microflora differences could influence survival and growth characteristics of pathogenic bacteria.
| |
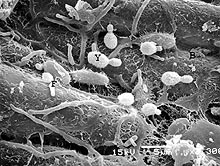
Fig. 2. It is largely unknown how the resident, non-pathogenic microflora affects the growth and survival of human pathogenic bacteria on raw fruits and vegetables. B = bacteria, F = fungi, Y = yeast. |
|
As with phytopathogenic bacteria (22), vegetable seed can support the prolonged survival and growth of human pathogens. Aerobic plate counts indicate that natural bacterial populations can reach levels of 10
4 colony forming units (CFU) per gram of alfalfa and onion seed (32). These populations vary significantly depending on how seed are produced, processed, and stored. Unfortunately, on sprout seed, small initial populations of pathogens can grow to high numbers during sprout production. Conditions of high relative humidity and temperature, together with nutrient-rich root exudates, support rapid bacterial multiplication. Populations of bacteria as high as 10
8 to 10
9 CFU/g have been observed on sprouted onion and alfalfa tissues (32).
Salmonella Stanley populations have been reported to increase by 2.5 log units within 24 h of germination for contaminated alfalfa seed (18). Pathogenic bacteria can survive for prolonged periods in or on stored dried seed, and long-term survival is greater at lower temperatures (43). Hence, finding the optimum storage conditions that promote the desiccation and ultimate reduction of bacterial populations without compromising seed quality is a viable option for reducing bacterial populations. Taormina and Beuchat (43) found that
E. coli O157:H7 populations on artificially infested alfalfa seed remained viable for up to 38 weeks when stored at 5°C. On the other hand,
E. coli O157:H7 populations declined rapidly after 1 week at 25°C or 37°C. After 8 weeks of storage at 37°C, the pathogen could no longer be recovered by direct plating but was recovered by a more sensitive enrichment technique. After 54 weeks,
E. coli O157:H7 could not be detected. It is unlikely that appropriate storage conditions alone could be a reliable method to eliminate pathogenic bacteria from infested seed; however, the right storage conditions could reduce the risk of cross-contamination. It is also critical to ensure that rodents and other animals are excluded from facilities to prevent fecal contamination of seed.
Physical seed damage, which may occur during seed conditioning, may promote seed deterioration and enhance bacterial survival and cross-contamination (43). Blending of seed of different origins for sprout production presents an additional risk of cross-contamination and makes it difficult to track the source of contamination in subsequent epidemiological investigations. Finally, improper storage of seed can lead to seed decay, which is usually accompanied by both qualitative and quantitative increases in the seed microflora.
Methods to Eliminate Human Pathogens from Fresh Produce
The lack of an effective antimicrobial treatment at any step from planting to consumption means that pathogens introduced at any point may be present on the final food product. Washing and rinsing some types of fruits and vegetables prolong shelf-life by reducing the number of microorganisms on the surfaces. However, only a portion of pathogenic microorganisms may be removed with this simple treatment. Use of a disinfectant can enhance efficiency of removal up to 100 fold, but chemical treatments administered to whole and cut produce typically will not reduce populations of pathogens by more than 2 to 3 log10 CFU/g (5). Pathogens also vary in their sensitivity to sanitizers. For example, L. monocytogenes is generally more resistant to chlorine than are Salmonella and E. coli O157:H7 (5). The general lack of efficacy of sanitizers on raw fruits and vegetables can be attributed, in part, to their inaccessibility to locations within structures and tissues that harbor pathogens. Pathogenic bacteria are able to infiltrate cracks, crevices, and intercellular spaces of seeds and produce. Infiltration is dependent on temperature, time, and pressure, and only occurs when the water pressure on the produce surface overcomes internal gas pressure and the hydrophobic nature of the surface of the produce (6,9). Infiltration may also be enhanced by the presence of surfactants and when the temperature of the fruit or vegetable is higher than the temperature of a water suspension of cells. The protective mechanism of these sites is not well understood but the concept that hydrophobicity of microbial cells aids in their protection by inhibiting penetration of the disinfectants has been proposed.
Seed Treatment
The elimination of bacteria from seeds by chemical or physical treatment is critical for reducing the risks of sproutborne disease outbreaks. In many of these outbreaks, vegetable seed sprouts have been implicated as the initial inoculum source. While they significantly reduce bacterial contamination, it is unlikely that chemical seed treatments can completely eliminate bacteria from seed. The biggest barriers to complete disinfestation of seed include: (1) treatment dosages must inactivate microorganisms without adversely affecting seed viability; (2) treatments must contact bacteria that in some cases are located in protected seed tissues; and (3) seed may inactivate certain treatments, making them less effective. Nevertheless, there have been many reports describing the efficacy of chemical seed treatments for sprout seed including chlorine compounds (e.g., calcium and sodium hypochlorite), ethanol, hydrogen peroxide, calcium EDTA, 4-hydroxybenzoic acid, ozonated water, and commercial disinfestants (4,5,8,19,43,50). Treatment of sprout seed with gaseous chemicals has also been evaluated (12,49). Similar to results observed with fresh produce, seed treatment fail to completely eliminate the pathogens on a consistent basis. This failure has been attributed to the inability of chemical disinfestants to make contact with the bacteria within protected seed tissues.
Thermotherapy (e.g., hot water treatment) is another option that has been explored for seed disinfestation (14). This approach includes exposing seeds to temperatures of 57 to 60°C for short periods (e.g., 10 min) (40) and has been employed extensively for seedborne phytopathogens with varying levels of success (14). Elevated temperatures will kill seedborne bacteria; however, the negative impact of hot water treatments on seed germination and sprout vigor is of great concern. There is a narrow optimum range for the temperature and exposure time that makes hot water treatments risky and difficult to implement commercially. For example, when treating large batches of seed, it is difficult to ensure uniformity of temperature throughout the water bath and, while a portion of the seed receives the appropriate temperature-time exposure, some will still contain viable bacteria after treatment. Additionally, there is the risk of cross-contamination using this practice. Combinations of thermotherapy with chlorine have also been shown to reduce, but not eliminate, populations of Salmonella (18) and E. coli O157:H7 (7) on alfalfa seeds.
Ionizing radiation has also been explored for the elimination of E. coli and Salmonella from seeds intended for sprouting (34). Radiation energy is a particularly attractive treatment because it can penetrate seed tissues and possibly eliminate bacteria localized within protected tissues. Irradiation of seeds also has been shown to reduce Salmonella populations without affecting germination (34). However, high levels of irradiation can negatively affect the physiology of seedlings and more research is needed to assess the potential and risks of this approach. Other non-thermal approaches, including super-critical carbon dioxide (pressurized CO2) (23), ultraviolet radiation, ultrasound treatments (40), and magnetic resonance fields, may have potential as seed treatments for reducing populations of foodborne pathogens.
In general, most of the sprout seed decontamination approaches reduce, but fail to completely eliminate, seedborne bacteria. Unfortunately, population reduction alone is unsatisfactory because even low bacterial populations pose significant public health risks due to explosive increases during sprouting. Similar to results observed with fresh produce, seed treatments fail to completely eliminate the pathogens on a consistent basis. However, greater success may be achieved by combining compatible seed treatments to yield disinfestation capabilities without compromising seed viability or physiology.
Seed Testing
As mentioned above, the ability to effectively determine the source of microbial contamination of seed is difficult. Seed testing is a requisite tactic for identifying and excluding sprout seeds as a source of contamination with pathogenic bacteria. However, testing seed for human pathogens is plagued by the same problems faced by testing for phytopathogenic bacteria. Namely, the number of contaminated seeds within naturally infested seedlots is usually low and unevenly distributed. Hence, statistically sound sampling schemes must be employed. Inadequate sampling or small sample sizes may explain why laboratory analyses of sprout seed samples have rarely led to the detection of Salmonella or E. coli O157:H7 in suspected lots. Nevertheless, seed assays should be rapid, sensitive, specific, and easy to interpret. Many seed detection assays are available for bacteria, including semi-selective agar media, serology-based techniques (ELISA and immuno-fluorescence staining), and culture enrichment (36); however, the processes by which bacteria are extracted from seeds are limited and sometimes inadequate. This is mainly due to the uneven surface features of seed coats that protect bacteria (10). This inability to efficiently extract bacteria contributes significantly to the variability observed in seed test performance. In general, bacteria are extracted from seeds by extended soaking in buffer or by physical disruption (e.g., in a stomacher or blender). Wu et al. (51) found that disrupting the seedcoat with a stomacher or blender increased the release of E. coli O157:H7 cells from artificially infested alfalfa seed. While 106 CFU of E. coli O157:H7 per gram of alfalfa seed were recovered, it may be possible to improve the detection of extracted bacteria by molecular-based assays such as the polymerase chain reaction (PCR) (13,38). Recently, PCR has evolved as a technique that is highly applicable for detecting pathogens and has vast potential for screening sprout seeds (39,47,48). Unfortunately, many seed types contain compounds that inhibit PCR, yielding false-negative results, and the development of test protocols to overcome these limitations is needed. One such modification is immunomagnetic separation (IMS) by which antibodies attached to microscopic polystyrene beads are used to sequester bacteria from heterogeneous suspensions (30,37) (Fig. 3). The captured cells can be rinsed and, after lysis, the DNA can be used in PCR analysis. IMS protocols have been reported for E. coli O157:H7 and have been used to recover target cells (51). Because of the difficulties associated with sampling and detecting bacteria in large seed lots, a negative seed test result does not necessarily indicate that pathogenic bacteria are not present. On the other hand, a positive seed test result can provide information to prevent the distribution and use of contaminated seed.
| |
Fig. 3. Schematic diagram of the immunomagnetic separation and polymerase chain reaction assay for the detection of bacteria in seed. Super paramagnetic beads (B) coated with antibodies specific to the target bacterium are incubated with seed extracts. Beads are then rinsed to eliminate non-target bacteria and PCR inhibitors then either boiled to extract DNA for PCR detection or plated on selective media for recovery of viable colonies (48). |
|
Plant Pathology and Foodborne Pathogens

Fig. 4. Onion seedling germinating with droplet of Pantoea ananatis on the emerging seedling (arrow). |
|
So what is the potential role of plant pathologists in better understanding the contamination of food crops by human pathogens and devising better management options to limit human infections? Plant surface microbiology has been actively studied for many years (e.g., 20), and much of the research is germane to human pathogenic bacteria on seed or fresh produce. Some research on the interactions between plant pathogens contaminating food and the growth and survival of human pathogens has been completed (e.g., 11,35), but more could be done. One impediment to this research is the lack of proper facilities (e.g., for biohazard safety) for working with human pathogens in plant pathology laboratories. Many parallels also exist between research in seed pathology and contamination of seed by human pathogens (Fig. 4). An exchange of research tools and experiences between seed pathologists and food microbiologists could result in tremendous advances towards the management of foodborne illness outbreaks associated with contaminated vegetables.
While it is unlikely that a single strategy will be successful in eliminating contamination of fresh produce and seed by human pathogenic bacteria, a multi-pronged approach, including sound regulatory policies with adequate enforcement, good agricultural practices in the seed production field, adherence to good manufacturing practices during minimal processing, proper harvesting and storage, seed testing, and antimicrobial treatments may reduce the risks of outbreaks of foodborne illnesses associated with fresh produce and vegetables. Such integrated pest management models have been implemented successfully for some plant diseases and it is likely that this approach will work for minimizing the risk of human pathogenic bacteria on seed and fresh produce.
Additional Resources
Centers for Disease Control and Prevention (CDC)
Foodborne Diseases Active Surveillance Network (FoodNet) from the CDC
Center for Food Safety and Applied Nutrition (CFSAN)
Bad Bug Book from the USDA and CFSAN
Gateway to Government Food Safety Information
Food Safety and Inspection Service (FSIS) of the USDA
Foodborne Illness Education Information Center of the USDA
Literature Cited
1. Altekruse, S. F., and Swerdlow, D. L. 1996. The changing epidemiology of foodborne diseases. Am. J. Med. Sci. 311:23-29.
2. Bean, N. H., Goulding, J. S., Daniels, M. T., and Angulo, F. J. 1997. Surveillance for foodborne disease outbreaks: United States, 1988 - 1992. J. Food Prot. 60:1265-1286.
3. Beuchat, L. R. 1996. Pathogenic microorganisms associated with fresh produce. J. Food Prot. 59:204-216.
4. Beuchat, L. R. 1997. Comparison of chemical treatments to kill Salmonella on alfalfa seeds destined for sprout production. Intl. J. Food Microbiol. 34:329-333.
5. Beuchat, L. R. 1998. Surface decontamination of fruits and vegetables eaten raw: A review. Food Safety Unit, World Health Organization. WHO/FSF/FOS/98.2.
6. Beuchat, L. R. 2002. Ecological factors influencing survival and growth of human pathogens on raw fruits and vegetables. Microbes Infect. 4:413-423.
7. Beuchat, L. R., and Scouten, A. J. 2002. Combined effects of water activity, temperature and chemical treatments on the survival of Salmonella and Escherichia coli O157:H7 on alfalfa seeds. J. Appl. Microbiol. 92:382-395.
8. Beuchat, L. R., Ward, T. E., and Pettigrew, C. A. 2001. Comparison of chlorine and a prototype produce wash product for effectiveness in killing Salmonella and Escherichia coli O157:H7 on alfalfa seeds. J. Food Prot. 64:152-158.
9. Burnett, S. L., and Beuchat, L. R. 2001. Human pathogens associated with raw produce and unpasteurized juices, and difficulties in contamination. J. Indust. Microbiol. Biotechnol. 27:104-110.
10. Charkowski, A. O., Sarreal, C. Z., and Mandrell, R. E. 2001. Wrinkled alfalfa seeds harbor more aerobic bacteria and are more difficult to sanitize than smooth seeds. J. Food Prot. 64:1292-1298.
11. Conway, W. S., Leverentz, B., Saftner, R. A., Janisiewicz, W. J., Sams, C. E., and Leblanc, E. 2000. Survival and growth of Listeria monocytogenes on fresh-cut apple slices and its interaction with Glomerella cingulata and Penicillium expansum. Plant Dis. 84:177-181.
12. Delaquis, P. J., Sholberg, P. L., and Stanich, K. 1999. Disinfection of mung bean seed with gaseous acetic acid. J. Food Prot. 62:953-957.
13. Erlich, H. A., Gelfand, D. H., and Saiki, R. K. 1988. Specific DNA amplification. Nature 331:461-462.
14. Grondeau, C., and Samson, R. 1994. A review of thermotherapy to free plant materials from pathogens, especially seeds from bacteria. Crit. Rev. Plant Sci. 13:57-75.
15. Guiterrez, E. 1997. Japan prepares as O157 strikes again. Lancet 349:1156.
16. Guo, X., van Iersel, M. W., Chen, J., Brackett, R. E., and Beuchat, L. R. 2002. Evidence of association of salmonellae with tomato plants grown hydroponically in inoculated nutrient solution. Appl. Environ. Microbiol. 68:3639-3643.
17. Hedberg, C. W., MacDonald, K. L., and Osterholm, M. T. 1994. Changing epidemiology of foodborne disease: A Minnesota perspective. Clin. Infect. Dis. 18:671-682.
18. Jaquette, C. B., Beuchat, L. R., and Mahon, B. E. 1996. Efficacy of chlorine and heat treatment in killing Salmonella Stanley inoculated onto alfalfa seeds and growth and survival of the pathogen during sprouting and storage. Appl. Environ. Microbiol. 62:2212-2215.
19. Lang, M. M., Ingham, B. H., and Ingham, S. C. 2000. Efficacy of novel organic acid and hypochlorite treatments for eliminating Escherichia coli O157:H7 from alfalfa seeds prior to sprouting. Intl. J. Food Microbiol. 58:73-82.
20. Lindow, S. E., Hecht-Poinar, E. I., and Elliot, V. J. 2002. Phyllosphere Microbiology. American Phytopathological Society, St. Paul, MN.
21. Mahon, B. E., Ponka, A., Hall, W. N., Komatsu, K., Dietrich, S. E., Siitonen, A., Cage, G., Hayes, P. S., LambertFair, M. A., Bean, N. H., Griffin, P. M., and Slutsker, L. 1997. An international outbreak of Salmonella infections caused by alfalfa sprouts grown from contaminated seeds. J. Inf. Dis.175:876-882.
22. Maude, R. B. 1996. Seedborne disease and their control: Principles and practice. CAB International, Wallingford, UK.
23. Mazzoni, A. M., Sharma, R. R., Demirci, A., and Ziegler, G. R. 2001. Supercritical carbon dioxide treatment to inactivate aerobic microorganisms on alfalfa seeds. J. Food Safety 21:215-223.
24. Mead, P. S., Slutsker, L., Dietz, V., McGaig, L. F., Bresee, J. S., Shapiro, C., Griffin, P. M., and Tauxe, R. V. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625.
25. Morris, C. E., Monier, J. E., Jacques, M. A. 1997. Methods for observing microbial biofilms directly on leaf surfaces and recovering them for isolation of culturable microorganisms. Appl. Environ. Microbiol. 63:1570-1576.
26. National Advisory Committee on Microbiological Criteria for Foods. 1999. Microbiological safety evaluations and recommendations on fresh produce. Food Control 10:117-143.
27. National Advisory Committee on Microbiological Criteria for Foods. 1999. Microbiological safety evaluations and recommendations on sprouted seeds. Int. J. Food Microbiol. 52:123-153.
28. Nguyen-the, C., and Carlin, F. 1994. The microbiology of minimally processed fresh fruits and vegetables. Crit. Rev. Food Sci. Nutr. 34:371-401.
29. Norwood, D. E., and Gilmour, A. 2000. The growth and resistance to sodium hypochlorite of Listeria monocytogenes in a steady-state multispecies biofilm. J. Appl. Microbiol. 88:512-520.
30. Olsvik, O., Popovic, T., Skjerve, E., Cudjoe, K. S., Hornes, E., Ugelstad, J., and Uhlen, M. 1994. Magnetic separation techniques in diagnostic microbiology. Clin. Microbiol. Rev. 7:43-54.
31. Proctor, M. E., Hamacher, M., Tortorello, M. L., Archer, J. R., and Davis, J. P. 2001. Multistate outbreak of Salmonella serovar Muenchen infections associated with alfalfa sprouts grown from seeds pretreated with calcium hypochlorite. J. Clin. Microbiol. 39:3461-3465.
32. Prokopowich, D., and Blank, G. 1991. Microbiological evaluation of vegetable sprouts and seeds. J. Food Prot. 54:560-562.
33. Puohiniemi, R., Heiskanen, T., and Siitonen, A. 1997. Molecular epidemiology of two international sprout-borne Salmonella outbreaks. J. Clin. Microbiol. 35:2487-2491.
34. Rajkowski, K. T., and Thayer, D. W. 2000. Reduction of Salmonella spp. and strains of Escherichia coli O157:H7 by gamma radiation of inoculated sprouts. J. Food Prot. 63:871-875.
35. Riordan, D. C. R., Sapers, G. M., and Annous, B. A. 2000. The survival of Escherichia coli O157:H7 in the presence of Penicillium expansum and Glomerella cingulata in wounds on apple surfaces. J. Food Prot. 63:1637-1642.
36. Saettler, A. W., Schaad, N. W., and Roth, D. A. 1989. Detection of bacteria in seed and other planting material. American Phytopathological Society, St. Paul, MN.
37. Safarik, I., and Safarikova, M. 1999. Use of magnetic techniques for the isolation of cells. J. Chromatogr. B 722:33-53.
38. Saiki, R. K., Gelfand, D. H., Stoffel, S., Scharf, S. J., Higuchi, R., Horn, G. T., Mullis, K. B., and Erlich, H. A. 1988. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 239:487-491.
39. Schaad, N. W., Cheong, S. S., Tamaki, S., Hatziloukas, E., and Panopuolos, N. J. 1995. A combined biological and enzymatic amplification (BIO-PCR) technique to detect Pseudomonas syringae pv. phaseolicola in been seed extracts. Phytopathology 85:243-248.
40. Scouten, A. J., and Beuchat, L. R. 2002. Combined effects of chemical, heat and ultrasound treatments to kill Salmonella and Escherichia coli O157:H7 on alfalfa seeds. J. Appl. Microbiol. 92:668-674.
41. Solomon, E. B., Yaron, S., Matthews, K. R. 2002. Transmission of Escherichia coli O157:H7 from contaminated manure and irrigation water to lettuce plant tissue and its subsequent internalization. Appl. Environ. Microbiol. 68:397-400.
42. Taormina, P. J., Beuchat, L. R., and Slutsker, L. 1999. Infections associated with eating seed sprouts: An international concern. Emerg. Infect. Dis. 5:626-634.
43. Taormina, P. J., and Beuchat, L. R. 1999. Comparison of chemical treatments to eliminate enterohemorrhagic Escherichia coli O157:H7 on alfalfa seeds. J. Food Prot. 62:318-324.
44. Taylor, E., Bates, J., Kenyon, D., Maccaferri, M., and Thomas, J. 2002. Modern molecular methods for characterisation and diagnosis of seed-borne fungal pathogens. J. Plant Pathology 83:75-81.
45. USDA. 1998. The guide at a glance: The guide to minimize microbial food safety hazards for fresh fruits and vegetables, in brief. Online. USDA Center for Food Safety and Applied Nutrition. Food Safety Initiative, HFS-32.
46. Wachtel, M. R., Whitehand, L. C., and Mandrell, R. E. 2002. Association of Escherichia coli O157:H7 with preharvest leaf lettuce upon exposure to contaminated irrigation water. J. Food Prot. 65:18-25.
47. Walcott, R. R., Gitaitis, R. D., Castro, A. C., Sanders, F. H., and Diaz-Perez, J. C. 2002. Natural infestation of onion seed by Pantoea ananatis, causal agent of center rot. Plant Dis. 86:106-111.
48. Walcott, R. R., and Gitaitis, R. D. 2000. Detection of Acidovorax avenae subsp. citrulli in watermelon seed using immunomagnetic separation and the polymerase chain reaction. Plant Dis. 84:470-474.
49. Weissinger, W. R., McWatters, K. H., and Beuchat, L. R. 2001. Evaluation of volatile chemical treatments for lethality to Salmonella on alfalfa seeds and sprouts. J. Food Prot. 64:442-450.
50. Weissinger, W. R., and Beuchat, L. R. 2000. Comparison of aqueous chemical treatments to eliminate Salmonella on alfalfa seeds. J. Food Prot. 63:1475-1482.
51. Wells, J. M., and Butterfield, J. E. 1997. Salmonella contamination associated with bacterial soft rot of fresh fruits and vegetables in the marketplace. Plant Dis. 81:867-872.
52. Wu, F. M., Beuchat, L. R., Wells, J. G., Slutsker, L., Doyle, M. P., and Swaminathan, B. 2001. Factors influencing the detection and enumeration of Escherichia coli O157:H7 on alfalfa seeds. Int. J. Food Microbiol. 71:93-99.
