Gary D. Franc
Professor
University of Wyoming
College of Agriculture, Plant Sciences Department-3354
1000 E. University Ave. (Shipping: 16th & Gibbon Streets)
Laramie, WY 82071
Tel: (307) 766-2397
FAX: (307) 766-5549
(Corresponding author: francg@uwyo.edu)
Franc, G. 2007 Potato Wart. Online. APSnet Features. doi: 10.1094/APSnetFeature-2007-0607
Introduction
Potato wart is an important and serious disease of cultivated potato (Solanum tuberosum) with numerous accounts of disease detections occurring worldwide [e.g., (1,4,6)]. Potato wart is known by various names, including black scab, black wart, cauliflower disease, potato tumor, potato cancer, potato canker, wart, warty disease, and certainly many other descriptive names in several languages. The disease is caused by the fungus Synchytrium endobioticum, and this organism is considered to be the most important world-wide quarantine plant pathogen of cultivated potato. S. endobiotocum is a primitive fungus characterized by the lack of hyphae, the formation of zoospores and the development of long-lived resting sporangia (13). This fungus invades certain meristematic tissues of the potato plant. This can preclude plant emergence when sprout tips are infected. After plant emergence, when stolon tips or tuber eyes become infected, resultant disease processes can render the potato tuber unrecognizable and unfit for human consumption. However, it should be noted that the potato wart fungus is not a human pathogen and poses no threat to human health.
Resting sporangia released from infected tissue can render soil unsuitable for potato production for decades (11). The long term survival of resting sporangia and the concomitant lack of suitable chemical controls for potato wart suppression make this disease especially problematic for any type of cultivated potato production, including potato production scales ranging in size from small garden plots and subsistence farming to extensive land areas economically dependent on the commercial production of potatoes for consumption or for seed potato production.
The potato wart pathogen is readily distributed via movement of infested soil and by infected seed tubers [e.g., (1,4,6)]. Because of the great potential for disease loss, any degree of infestation or indication of potato wart universally (world-wide) results in strict quarantine and other regulatory measures designed to confine the known infestation and to preclude pathogen re-distribution. Although direct losses from potato wart may be insignificant when first detected, indirect economic losses resulting from zero-tolerance regulations for potato wart can be devastating to the affected parties. Indirect economic losses become especially evident in situations where seed tuber production areas become subject to quarantine measures, as well as when the movement of commercial potatoes is restricted. For example, a well-documented detection of potato wart on Prince Edward Island (Canada) late in the 2000 growing season and the ramifications of subsequent regulatory actions resulted in an estimated $30 million loss to the Island’s economy in that first year alone. Visit AgNet reports for the local and national perspective pertaining to the far-reaching economic, social and political impacts that resulted from a single detection (at that time) of potato wart in a small portion of one potato field.
Distribution
The potato wart pathogen co-evolved with the potato in the Andes Mountains in South America from where it was distributed around the world by infected tubers and infested soil [see summary by Hampson (8)]. The pathogen is believed to have been first introduced to the European community with potato breeding material from the South American Andes brought in during the aftermath of the 1840-1850 potato late blight tragedy, a disease caused by Phytophthora infestans. By one historical account, potato wart was reported to enter England in 1876 or 1878, and another account stated that "the disease" had been known to exist in the Liverpool district of England prior to 1893. Potato wart was then thought to have spread to most potato growing areas in Europe, being first reported on the European continent in Czechoslovakia (1888), followed by one of the earliest formal descriptions of potato wart and the pathogen (by Schilbersky) from upper Hungary in 1896. In 1908, potato wart was reported from Ireland and Germany and shortly afterwards in Scotland and Wales. The disease was first found in the Netherlands around 1914, and gradually spread into other potato-growing regions of Europe until the World War II.
The first report of potato wart from North America was in 1909 in Newfoundland, Canada. In 1910, the United States Department of Agriculture (USDA) issued warnings about importing wart-affected potatoes and, in 1912 the Federal Horticulture Board (United States) enacted an embargo of potatoes coming from countries where potato wart was known to exist (10). Although well-intentioned, several million bushels (1 million bushel = 60 million lb = 2.72 x 104 metric tons) of the 1911 potato crop had already been imported to the United States from Europe, and several smaller importations had occurred in previous years prior to the embargo. In 1918, potato wart was first reported to be present in the United Sates when it was found in small garden plots located in 27 villages and cities in Pennsylvania (10). It was subsequently believed that the disease had been present in the United States for at least four years prior to this first report.
Potato wart has now been variously reported in Asia, Africa, Europe, Oceania, North America, and South America (4). Summaries of potato wart distribution are available from different sources. However, this information must be carefully reviewed for accuracy because it becomes obsolete as new disease detections are made, and also because situations occur where regulatory actions have resulted in successful eradication of the pathogen and an area is documented to be free from potato wart.
Although worldwide distribution of potato wart has seemingly occurred, these reports fail to indicate the truly fragmented nature of potato wart distribution that occurs in most countries. Fragmented distribution is attributed to the early (i.e., historical) recognition that potato wart was a potentially devastating disease and that strict regulatory actions were needed to limit movement of the pathogen [e.g., (5,10)]. These regulatory actions plus the absence of natural dispersal mechanisms by the fungus have greatly aided efforts to isolate and confine the pathogen to the site(s) where potato wart was initially detected. Therefore, although pockets of infestation are globally distributed, rigorous regulatory measures have prevented or limited general distribution of the pathogen beyond many of these foci.
Symptoms and Pathogen Identification
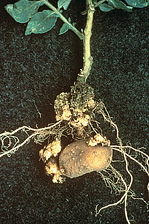
Fig. 1. Belowground portions of the potato plant are often infected by the potato wart pathogen. Following infection by the potato wart pathogen, meristematic tissues are transformed into large warty galls on the lower stems and tubers.
|
|
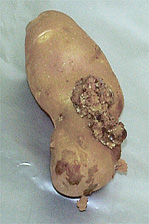
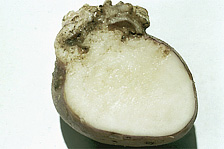
The diagnostic symptoms of potato wart are galls produced on several plant parts. These galls are primarily parenchymatous and may form on stem tissue, including the stem base, stolon buds, and tuber eyes. Galls most often form on below-ground portions of the plant (Fig. 1), and the presence of the disease is typically not noticed until tubers are lifted during harvest (Figs. 2 and 3). However, galls also occasionally form on the upper stem, leaf, or flower. Galls will vary in shape but are mostly spherical outgrowths, ranging from 1 to 8 cm (0.4 to 3.2 inches) or more in diameter, but may become fist-sized in some instances. Aboveground galls are green to brown, turning black at maturity, and are prone to decay. Belowground galls are white to brown and turn black as they decay (Fig. 4). Tubers may be disfigured or become almost unrecognizable when they are infected early in development and replaced by galls. Galls present on tubers at harvest may become desiccated and barely noticeable or, alternatively, the galls may decay. Because the disease can continue developing during storage, desiccated galls barely noticed at harvest may become increasingly evident during prolonged storage. Although potato wart does not kill the host, the meristematic tissue of sprouts may be so severely attacked that plants ultimately fail to emerge from seed tubers early in the growing season. Also, infected plants may develop general symptoms of reduced vigor, especially in association with the formation of small greenish-yellow warty growths at the stem base. The true potato roots are not known to be infected (e.g., Fig. 1).
| |
Fig. 2. The presence of potato wart often goes unnoticed until tubers with warty galls are detected during harvest. (Photo by L. Ward from the APS digital image collection CD Diseases of Root and Tuber Crops 2002.) |
|
 |
|
|
Fig. 3. A cross-section of a potato wart gall on a tuber reveals that galls result from an overgrowth of tuber tissue. The potato wart pathogen reproduces within gall tissue. (Photo by M.C. Hampson from the APS digital image collection CD Diseases of Root and Tuber Crops 2002.) |
|
 |
| |
Fig. 4. Potato wart galls on tubers initially appear white, and then turn brown or black as they begin to decay. (Photo by L. Ward from the APS digital image collection CD Diseases of Root and Tuber Crops 2002.) |
|
 |
|
Symptoms associated with potato wart may appear similar to some of the symptoms caused by powdery scab (
Spongospora subterranea f. sp.
subterrenea), potato smut (
Thecaphora solani), and ‘pseudo-wart,’ a proliferation of eyes that may be a physiological or varietal response or, alternatively, induced by chemical factors (1). Therefore, proper identification and laboratory confirmation is needed to identify the presence of the potato wart pathogen (1). As an aid to identification, PCR-based methods are being developed for both detection and quantification of
S. endobioticum in soil and host tissues (12). Because
S. endobioticum is on the United States Federal Select Agent list, any suspected incidence(s) of the pathogen in the United States must be verified by sending samples to the Plant Protection and Quarantine division of APHIS for confirmation by the National Mycologist.
Causal Organism
Synchytrium endobioticum (Schilberszky) Percival does not produce hyphae and is an obligate, holocarpic, endobiotic parasite (8,13). It is a long-cycled chytrid characterized by a short-lived swarm (summer) sporangial stage resulting from host infection by uniflagellate zoospores and a resting (winter) sporangial stage resulting from host infection by conjugated zoospores (biflagellate zygotes). Both sporangial types germinate to release 200-300 zoospores, which are pear-shaped infective units (1.5 to 2.2 µm in diameter) and motile by means of a posterior flagellum. Resting sporangia are golden brown and spheroidal (35 to 80 µm in diameter). The resting sporangium wall has prominent exterior ridges and contains chitin fibrils.
Numerous pathotypes of the fungus exist and are defined by their virulence on differential potato cultivars. Approximately 43 pathotypes have been described for Europe, and these pathotypes presumably persist mainly in small garden potato plots, and not in commercial potato crops (3). The true number of pathotypes is not evident and their potential roles in disease development are poorly defined for most pathotypes because researchers in different countries have used various sets of differential cultivars for pathotype identification and characterization (3).
Disease Cycle
In the spring, resting sporangia in decaying warts and soil germinate to release haploid (uninucleate) zoospores (6,8). These zoospores migrate in soil water for a limited distance (50 mm or less) (2 inches or less) via a single flagellum to arrive at epidermal cells of meristematic tissues of growing points, buds, stolon tips, or young leaf primordia. Zoospores are short-lived and must encyst and infect susceptible host tissue within 1-2 hr after their formation. After infection by zoospores, potato host cells greatly enlarge and haploid sori form inside the host cells while neighboring host cells begin to proliferate, resulting in the characteristic warty galls and the increased presence of the meristematic tissue that provides new infection courts for the fungus. Each sorus contains one to nine summer sporangia, which in turn germinate to produce new haploid zoospores which reinfect susceptible tissue (i.e., a secondary disease cycle). These rapidly repeating secondary disease cycles ultimately result in an extensive invasion of host cells and rapid onset of gall formation. Young galls are a nutrient sink and expand rapidly at the expense of other plant tissue. For example, gall volume has been observed to increase more than 1,800-fold in 16 days (14).
Under conditions of stress, such as water shortage, zoospores also may conjugate (fuse) in pairs to form uninucleate, diploid, biflagellate zygotes which infect the host tissue to form resting sporangia. Following infection by zygotes, the host cell in which resting sporangia form does not swell but divides to form galls. The host cell wall remains closely attached and forms an outer layer to the resistant, thick-walled resting (winter) sporangium. As these galls decay and disintegrate, they release the thick-walled resting sporangia into the soil environment. Resting sporangia are endogenously dormant and can remain viable for 40 to 50 years at depths of up to 50 cm (20 inches) in the soil profile.
The resting sporangia are primarily spread in infected seed tubers, which may have incipient warts that pass undetected, or in infested soil adhering to tubers, equipment, and by other carriers of contaminated soil. Resting sporangia survive passage through the digestive system of animals fed infected potatoes, and contaminated manure also can disperse inoculum. Earthworms have also been found to serve as means of inoculum dispersal, and resting sporangia can be dispersed by wind-blown soil or by flowing surface water. The conventional wisdom is that zoospores are too short-lived to significantly contribute to inter-field dispersal of the potato wart pathogen. Most potato wart detections have been recorded in, or eventually traced to, small garden plots where there has been repeated culture of susceptible potato hosts.
Under ideal conditions, potato wart can develop when the inoculum density is less than one resting spore per gram of soil (7). Potato wart is favored by cool, wet soils during tuber development. A soil temperature of at least 8°C (46°F) and water is required for the germination of both winter and summer sporangia and for the dispersal of zoospores (8). Cool summers with average temperatures of 18°C (64°F) or less, winters of approximately 160 days at or below 5°C (41°F), and annual precipitation of 70 cm (28 inches) are important for the development of the disease, and disease is generally limited to environments with these characteristics. Soil pH is of less importance; the disease has been found to occur in plants growing in soils ranging from pH 3.9 to pH 8.5. Temperatures of 12 to 24°C (54 to 75°F) favor infection.
Potato is the principal host of the pathogen. However, S. endobioticum has been experimentally transferred to several other members of the family Solanaceae, including tomato and nightshade. However, plants other than potato are not believed to be of importance in the disease cycle. Resistance has been found in several wild potato species and resistant cultivated species have been developed. Disease severity is influenced greatly by environmental conditions, the pathotype present, and host reaction to the pathogen (resistance).
Management
Resistant potato cultivars have been developed in Europe and North America. Resistant plants may become infected, but symptom development is suppressed. Galls on resistant plants remain superficial and scab-like, or (in some cultivars) zoospores of the pathogen are killed by a hypersensitive reaction of the infected host-plant tissue. A set of images illustrating the range of host reaction to infection by the potato wart pathogen is available online (4). The emergence of different S. endobioticum pathotypes has compromised the efficacy of host plant resistance (2).
Amendment of infested soil with crushed crab shell (23% chitin) has been found to suppress the disease in some situations (9). The mechanism of disease suppression is not known with certainty, but may be related to changes in soil microflora activity following incorporation of crushed crab shell or may be related to physiological changes in the host. No other chemical control is known that is not also damaging to either the soil or crops.
Worldwide prevention is based on the control of disease spread and pathogen exclusion via regulatory action. Regulatory action has largely restricted the spread of potato wart within potato growing regions, as the seriousness of the disease was quickly recognized. Once potato wart is detected, regulations generally prohibit potato production on infested soil, and also attempt to preclude soil movement from infested sites by any means. Examples include prohibiting growth of any plants destined for transplant (because they can carry infested soil) and requiring a continuous "cover crop" to reduce movement of inoculum via wind-blown soil. A safety zone around the infestation often is established where, if potato growth is not already prohibited, only resistant cultivars may be grown for crop consumption. There is a universal zero tolerance (no disease permitted) for production of the seed potato crop. Long-term survival of spores is problematic and makes regulatory action difficult. The efficacy of quarantines, levels of contamination, and pathogen distribution can be monitored with quantitative soil extraction techniques and by soil bioassay utilizing susceptible cultivars (4). Long-term monitoring of infested sites has enabled measuring the prolonged survival of resting sporangia, as well as enabled documenting successful eradication efforts resulting in freedom from potato wart.
Some regulations in certain situations permit financial compensation to producers for losses resulting from compliance with potato wart regulations (e.g., Canadian Food Inspection Agency). Compensation may be provided due to loss of the use of land to grow potatoes, costs associated with cleaning and disinfestation of equipment and facilities, as well as the disposal of affected potato tubers or other agricultural products. This approach to regulation is hoped to encourage reporting and compliance with quarantine actions by easing economic impact. In the United States, federal crop insurance compensation is generally provided for potato losses due to plant disease, provided that good farming practices were followed during production of that crop (see USDA-RMA Crop Policies).
The European and Mediterranean Plant Protection Organization (EPPO) lists Synchytrium enobioticum as an A2 quarantine pest (4), and the United States Department of Agriculture has the fungus on its official list of select agents and toxins. Therefore, any cultures and research with this pathogen is tightly regulated and requires specific permits. The United States National Plant Disease Recovery System, called for in Homeland Security Presidential Directive Number 9, requires the preparation of disease-specific documentation to insure that the tools, infrastructure, communication networks, and the capacity for mitigating the impact of high consequence plant diseases exists, so that a reasonable level of crop production is maintained. The draft recovery plan for potato wart disease is available online.
In summary, potato wart is much easier to prevent than it is to control. Therefore, there is great incentive to prevent the introduction of the potato wart pathogen to production areas, and, where it is already introduced, to limit its spread. Although regulatory action will always be a key component in the first line of defense for potato wart management, it is essential that research efforts continue with the goal of developing and improving reliable and integrated disease suppression methods to directly deal with this disease.
Additional Resources
from European and Mediterranean Plant Protection Organization
Data sheets on Synchytrium endobioticum, images of symptoms and signs, disease distribution, pathogen detection and diagnostics:
List of pests recommended for quarantine
Data Sheet (PDF)
Distribution Map
Diagnostic protocols (PDF)
Photos
from USDA-APHIS
Plant Health/Plant Pest Information
from National Agricultural Pest Information System (NAPIS)
Links for information on potato wart
from North American Plant Protection Organization (NAPPO)
Phytosanitary Alert System
from Canadian Food Inspection Agency (CFIA)
Biology, distribution, and identification of potato wart symptoms
Description of Canadian potato varieties
from the UK Department for Environment, Food and Rural Affairs (DEFRA)
Descriptions and photos of symptoms and information on measures taken against potato wart
from USDA-APHIS
Pest Lists
from the Canadian Phytopathological Society
Information on potato wart
Selected References
1. Anonymous. 2004. Diagnostic protocols for regulated pests: Synchytrium endobioticum. EPPO Bull. 34:213-218.
2. Baayen, R. P., Bonthuis, H., Withagen, J. C. M., Wander, J. G. N., Lamers, J. L., Meffert, J. P., Cochius, G., van Leeuwen, G. C. M., Hendriks, H., Heerink, B. G. J., van den Boogert, P. H. J. F., van de Griend, P., and Bosch, R. A. 2005. Resistance of potato cultivars to Synchytrium endobioticum in field and laboratory tests, risk of secondary infection, and implications for phytosanitary regulations. EPPO Bull. 35:9-23.
3. Baayen, R. P., Cochius, G., Hendriks, H., Meffert, J. P., Bakker, J., Bekker, M., van den Boogert, P. H. J. F., Stachewicz, H., and van Leeuwen, G. C. M. 2006. History of potato wart disease in Europe – a proposal for harmonisation in defining pathotypes. Euro. J. Plant Pathol. 116:21-31.
4. EPPO. 2006. A2 list of pests recommended for regulation as quarantine pests (version 2006-09). Online. Quarantine info. European and Mediterranean Plant Protection Organization (EPPO), Paris, France.
5. EPPO/CABI. 1992. Synchytrium endobioticum. Pages 638-642 in: Quarantine Pests for Europe. CAB International, Wallingford, England.
6. Franc, G. D. 2001. Wart. Pages 46-47 in: Compendium of Potato Diseases. W. R. Stevenson, R. Loria, G. D. Franc, and D. P. Weingartner, eds. American Phytopathological Society, St. Paul, MN.
7. Hampson, M. C. 1992. A bioassay for Synchytrium endobioticum using micropropagated potato plantlets. Can. J. Plant Pathol. 14:289-292.
8. Hampson, M. C. 1993. History, biology and control of potato wart disease in Canada. Can. J. Plant Pathol. 15:223-244.
9. Hampson, M. C., and Coombes, J. W. 1995. Reduction of potato wart disease with crushed crabshell: Suppression or eradication? Can. J. Plant Pathol. 17:69-74.
10. Kunkel, L. O. 1919. Wart of potatoes: a disease new to the United States. Office of Cotton, Truck, and Forage Crop Disease Investigations, Circ. 6.
11. Laidlaw, W. M. R. 1985. A method for the detection of resting sporangia of the potato wart disease (Synchytrium endobioticum) in the soil of old outbreak sites. Potato Res. 28:223-232.
12. van den Boogert, P. H. J. F., van-Gent-Pelzer, M. P. E., Bonants, P. J. M., De Boer, S. H., Wander, J. G. N., Lévesque, C. A., van Leeuwen, G. C. M., and Baayen. R. P. 2005. Development of PCR-based detection methods for the quarantine phytopathogen Synchytrium endobioticum, causal agent of potato wart disease. Euro. J. Plant Pathol. 113:47-57.
13. Walker, J. C. 1983. Synchytrium endobioticum. CMI Descriptions of Pathogenic Fungi and Bacteria, No. 755. CAB Int'l., Wallingford, England.
14. Weiss, F. 1925. The conditions of infection in potato wart. Am. J. Bot. 12:413-443.
