Hoefnagels, M.H. and B.D. Mason. 2016. Pecan Scab. The Plant Health Instructor. DOI: 10.1094/PHI-I-2016-0620-01
Pecan scab
Fusicladium effusum (former names include Fusicladosporium effusum, Fusicladium caryigenum, Cladosporium caryigenum, and Cladosporium effusum)
Host species include pecan (Carya illinoinensis) and other Carya species, including bitter pecan (C. aquatica), bitternut hickory (C. cordiformis), pignut hickory (C. glabra), shagbark hickory (C. ovata), and mockernut hickory (C. tomentosa). Pecan scab is widespread in the southeastern United States but has also been reported in other parts of the United States and in Canada, Mexico, Central America, South America, and New Zealand.
Authors
Mariëlle H. Hoefnagels1 and Bonnie D. Mason2
1Associate Professor, Department of Microbiology and Plant Biology, University of Oklahoma
2Former student, Department of Microbiology and Plant Biology, University of Oklahoma

Nuts infected with pecan scab.
Signs and Symptoms
Symptoms of infection are similar on all parts of an infected plant. Pecan scab affects the leaves, shucks, and twigs of the infected plant and manifests as small (1 to 5 mm), circular, black or olive green lesions (Figure 1). Less commonly, catkins and dormant buds may be affected. Lesions may coalesce and form larger blackened areas. In the early stages of infection, the lesions appear velvety, due to production of conidia on their surface. As the infection progresses the lesions harden and turn a dark grey to silvery-brown color, and can become dry, crack, and drop out of the leaf. Lesions on young shoots appear sunken due to the swelling of the tissue at the margins of the lesions (Figure 2). These lesions may persist for several seasons after the initial infection. Similar lesions occur on the nut shucks (Figure 3). These lesions may be slightly raised. A black, velvety, cushion-like mass called a stroma (plural: stromata) that forms in these lesions provides the basis for overwintering, and gives rise to reproductive structures called conidiophores in the following spring.

Figure 1 |

Figure 2 |

Figure 3 |
Pecan scab affects nuts in several ways. If severe, infection can result in defoliation and a reduction of the size and quality of the nut; if the infection occurs early in nut development, the nuts will abort. In addition, if F. effusum reaches deep tissues in the shuck, it can cause the shuck to cling to the shell of the nut (a condition called “stick-tight”). Separating the nut from the shell is impossible, which is problematic for shelling. The surface of severely infected nuts may also crack, giving a point of entry for secondary infections. In particular, the pink mold fungus, Cephalothecium roseum, can invade old lesions on the shucks.
Pathogen Biology
Fusicladium effusum is an ascomycete fungus in the class Dothideomycetes. Dothideomycetes reproduce both sexually and asexually, but only the asexual phase has been observed in F. effusum. Asexual spores (conidia) initiate infection on susceptible host tissue. Conidia may be present in simple or branched chains two to nine cells long, with individual cells being 10–24 μm long × 5–10 μm wide (Figure 4). Conidia are light brown, clavate, fusiform, ovate or almost cylindrical in shape. Under favorable conditions, these spores germinate and form germ tubes that penetrate the host tissue. Germ tubes may enlarge to form an appressorium immediately adjacent to the conidium, or elongate and form an appressorium distally. The appressoria penetrate the cuticle of the host tissue and initiate subcuticular hyphal growth. Conidiophores arise from the hyphae and rupture the cuticle, forming the visible part of the lesion on the tissue surface. Conidiophores may be solitary structures or form loose fascicles. Conidiophores are long, unbranched or apically branched cells, septate and pale to dark brown, and 22-130 × 4-6 µm in size. Conidia are discharged from the tips of the conidiophores and are dispersed by wind and rain.
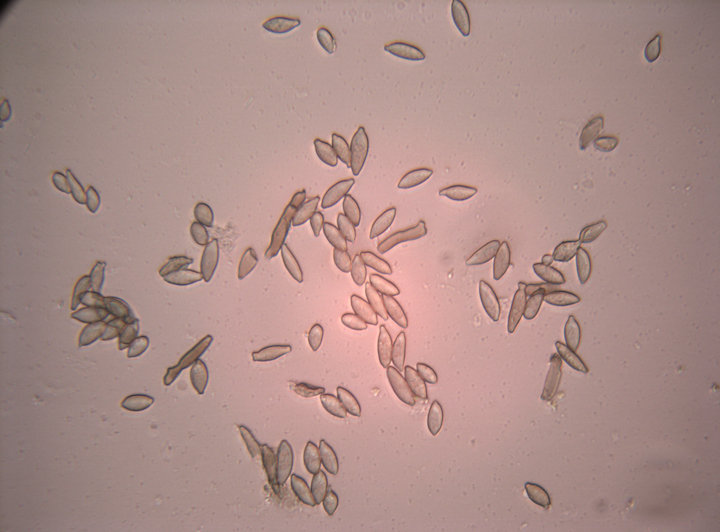
Figure 4 |
Disease Cycle and Epidemiology
Fusicladium effusum overwinters in lesions on the leaves, shucks, and twigs from the previous year’s infection ( Figure 5). These overwintering sites serve as the primary source of inoculum, while the current year’s infections produce lesions that act as a secondary source of inoculum and provide the polycyclic dimension to the disease.
|
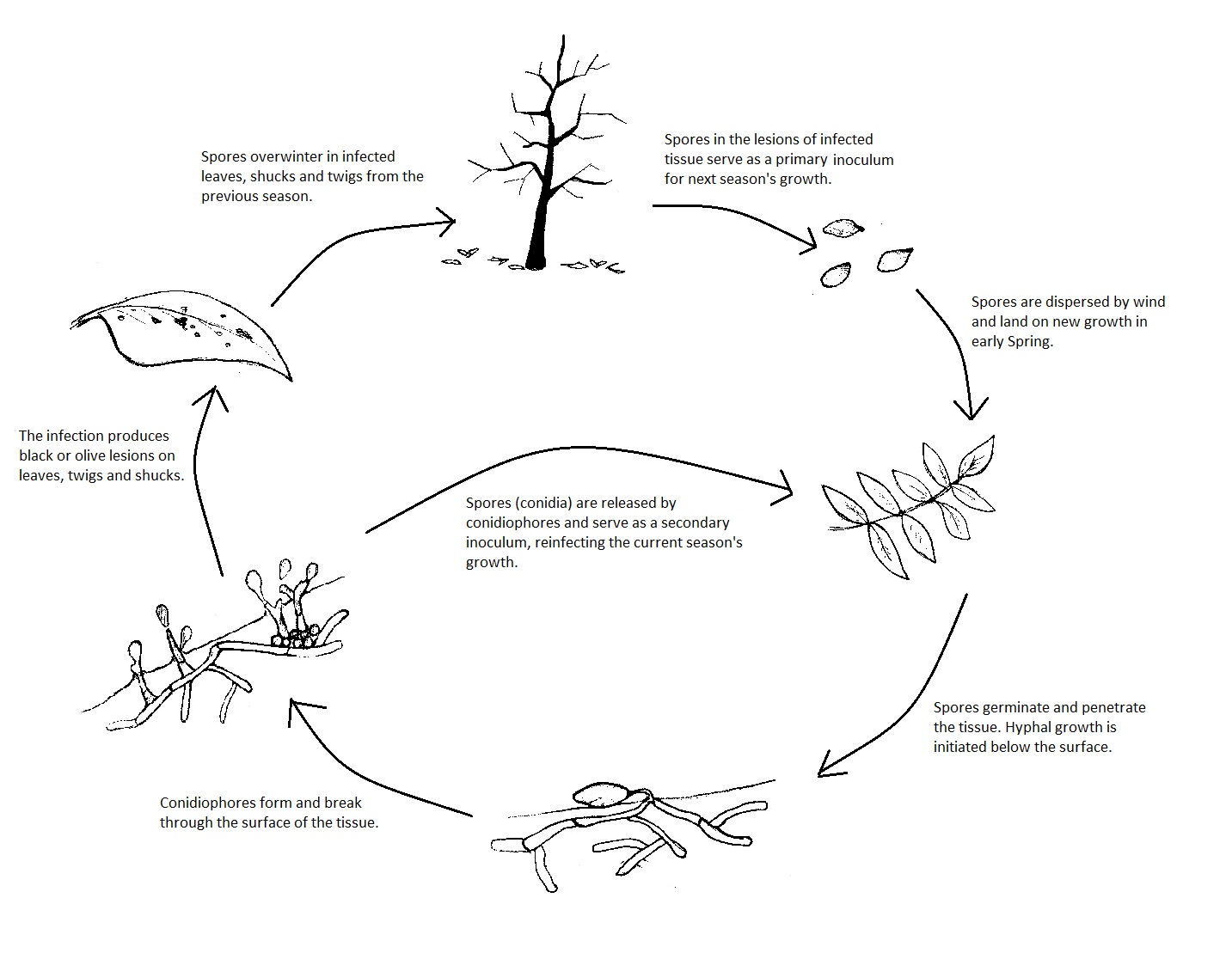
Figure 5
|
In spring, stromata in overwintering lesions begin producing conidiophores. Spore discharge is stimulated by sunlight and a rapid decrease in relative humidity and vegetative wetness. Spores are dispersed by wind as early as March and as late as December, with aerial spore concentrations being the highest in May and June. The conidia may land on developing nuts, newly emerging leaves, and twigs.
Surface moisture is critical for infection, and the optimum temperature range for infection is 20-30° C. Under favorable conditions, germination of conidia occurs within 3-24 h of inoculation. By 36 h post inoculation, the germ tube has typically penetrated the epidermis. Once the cuticle of the tissue is penetrated, hyphal growth occurs laterally within the tissue (Figure 5). Lesions appear 7-9 days after inoculation and the number of lesions increases with more prolonged leaf surface wetness. Conidiophores grow upward from the mycelial mass below the tissue surface and penetrate the epidermis (Figure 5). These conidiophores produce the conidia that act as a secondary inoculum throughout the growing season.
The pathogen primarily infects young, expanding leaves and shoots. The resulting lesions remain visible on older leaves; once leaves are fully expanded, however, they are effectively resistant to new infection. Fruit remain susceptible throughout their development and maturation. Therefore, infections established early in the season tend to be more severe and result in greatest crop loss.
Significance
Pecan scab can have a significant economic impact due to crop yield reduction and loss in quality. The disease causes nut drop, with total crop loss possible in severe cases. Reduction in the size and quality of the nuts occurs (Figure 6). In addition, pecan scab lesions on foliage reduce the photosynthetic area of the tree, causing a reduction in the photosynthetic rate of the plant. This reduction, in turn, may enhance alternate bearing, which is the tendency to produce a heavy crop one season, followed by one or more years of little or no production.
|

Figure 6
|
In addition to crop losses, pecan growers incur considerable expenses for costly preventative fungicides. As described in the next section, fungicides must be applied repeatedly throughout the growing season.
Management
Host resistance
The first line of defense against pecan scab is the selection of resistant cultivars. Cultivars may be rated on a scale of 1 to 5, with 1 meaning “no incidence of scab” and 5 meaning “very severe incidence.” Recommendations vary by region. For example, in old orchards in the southeastern United States, the Alabama Cooperative Extension lists Elliott (scab susceptibility rating = 1.4) and Davis (1.7) as the most scab-resistant cultivars; for new plantings, Jubilee (1.1) and Melrose (1.1) are even more resistant than Elliott, and Gloria Grande also shows good resistance (1.4). For commercial orchards in Georgia, the University of Georgia recommends Elliott and Kanza for excellent resistance and Sumner for good resistance; Gloria Grande is “not recommended for most situations.” This variation in cultivar resistance may reflect the existence of regional strains and high genetic variability in F. effusum.
Chemical control
Thanks to the historic loss of host resistance to F. effusum, commercial growers rely on fungicides to manage pecan scab. Organic growers may spray trees with the Bordeaux mixture (copper sulfate and hydrated lime). This fungicide was commonly used on pecans in the 1920s but was eventually supplanted in commercial pecan orchards by modern fungicides.
Today, multiple fungicides are used to control pecan scab. Overall, these chemicals inhibit spore germination or hyphal growth, kill germinating spores, or prevent sporulation. The Fungicide Resistance Action Committee organizes fungicides by mode of action at the cellular level. Chemicals that are typically used in commercial pecan production include benzimidazoles (mitosis/cell division inhibitors), strobilurins and organometals such as tri-phenyl tin compounds (respiration inhibitors), and triazoles (sterol biosynthesis inhibitors). Other fungicides used on pecans include dithiocarbamates (“multi-site contact activity”) and guanidines and phosphorous acid (phosphites), both of which have unknown modes of action. Optimal timing varies among these fungicides. For example, in one study, phosphites protected foliage early in the growing season but did not deliver late-season protection.
The Fungicide Resistance Action Committee also classifies fungicides according to their risk of selecting for resistant fungi. Dithiocarbamates and phosphorous acid (phosphites) are assigned to the low-risk category; guanidines and organometals are assigned a low to medium risk; triazoles have a medium risk for resistance; and benzimidazoles and strobilurins have a high risk. Consecutive application or too many applications of the same fungicide in a growing season may exacerbate pathogen resistance. For example, the Alabama Cooperative Extension recommends mixing triazole fungicides with organometals or alternating triazoles with organometals to reduce the selection pressure for resistant fungi.
Large, commercial air-blast sprayers are needed to ensure adequate coverage of fungicides, but even these sprayers may not be sufficient to reach the foliage and fruits in very tall trees (Figure 7). Inadequate coverage is a concern because low doses of fungicides may accelerate the selection for resistant varieties of F. effusum. Growers should begin spraying susceptible cultivars in early spring, when developing leaves are first exposed to inoculum from the overwintered lesions. Between bud break and nut set, fungicides should be applied every 10 to 14 days; from nut set to shell hardening, fungicides should be applied every 10 to 21 days. More frequent spraying may be required when conditions favor disease development. Multiple online tools analyze weather patterns and help growers determine if and when they should spray. For example, the Oklahoma Mesonet has an online Pecan Scab Advisor, and the AU-Pecan spray advisory is available to growers in Alabama, Georgia and Louisiana.
|

Figure 7 |
Cultural control
When establishing new orchards, tree spacing and orientation are important considerations, because adequate exposure to sunlight and good airflow are two keys to keeping foliage dry. Selective pruning of damaged branches during the dormant season is also recommended to promote sun exposure and air circulation. Finally, good sanitation practices are recommended to limit the amount of primary inoculum that may cause infection.
Acknowledgments
We thank Dr. William Reid (Kansas State University), Dr. Clive Bock (USDA-ARS), and Dr. Katherine Stevenson (University of Georgia) for their generosity in granting permission to use their images in this article. We also thank two anonymous reviewers for their helpful suggestions.
Selected References
Alabama Cooperative Extension. 2005. Commercial Pecan: Insect, disease, and weed control recommendations for 2005. Publication 2005IPM-27.
Bock, C. H. 2013. Fusicladium effusum (pecan scab). CABI Invasive Species Compendium.
Bock, C.H., Brenneman, T. B., Hotchkiss, M. W., and Wood, B. W. 2012. Evaluation of a phosphite fungicide to control pecan scab in the southeastern USA. Crop Protection 36:58-64.
Bock, C.H., Cottrell, T. E., Hotchkiss, M. W., and Wood, B. W. 2013. Vertical distribution of scab in large pecan trees. Plant Disease 97: 626-634.
Bock, C.H., Wood, B. W., Stevenson, K. L., and Arias, R. S. 2014. Genetic diversity and population structure of Fusicladium effusum on pecan in the United States. Plant Disease 98: 916-923.
Brenneman, T., Brock, J., Culpepper, A. S., Hudson, W., Mitchem, W., and Wells, L. 2015. Commercial Pecan Spray Guide. University of Georgia Cooperative Extension Service Bulletin No. 841. Demaree, J. B. 1924. Pecan scab with special reference to sources of the early spring infections. Journal of Agricultural Research 28: 321-330.
Demaree, J. B. 1928. Morphology and taxonomy of the Pecan-scab fungus, Cladosporium effusum (Wint.) comb. nov. Journal of Agricultural Research 37:181-187.
Demaree, J. B., and Cole, J. R. 1926. Commercial control of pecan scab. United States Department of Agriculture Department Circular 386. Farr, D.F., and Rossman, A.Y. Undated.
Fungal Databases – Nomenclature. Systematic Mycology and Microbiology Laboratory, ARS, USDA. Retrieved December 21, 2015, from
Fungicide Resistance Action Committee (FRAC). 2016. FRAC Code List 2016: Fungicides sorted by mode of action (including FRAC Code numbering).
Gottwald, T. R. 1982. Spore discharge by the pecan scab pathogen, Cladosporium caryigenum. Phytopathology 72: 1193-1197.
Gottwald, T. R. 1982. Taxonomy of the pecan scab fungus Cladosporium caryigenum. Mycologia 74:382-390.
Gottwald, T. R. 1985. Influence of temperature, leaf wetness period, leaf age, and spore concentration on infection of pecan leaves by conidia of Cladosporium caryigenum. Phytopathology 75:190-194.
Gottwald, T. R., and Bertrand, P. F. 1982. Patterns of diurnal and seasonal airborne spore concentrations of Fusicladium effusum and its impact on a pecan scab epidemic. Phytopathology 72:330-335.
Gottwald, T. R., and Bertrand, P. F. 1983. Effect of time of inoculation with Cladosporium caryigenum on pecan scab development and nut quality. Phytopathology 73: 714-718.
Gottwald, T. R., and Wood, B. W. 1985. Decreased net photosynthetic and dark respiration rates of pecan fruit and foliage in response to infection by Cladosporium caryigenum. Plant Disease 69:800-803.
Hunter, R. E. 1983. Influence of scab on late season nut drop of pecans. Plant Disease 67:806-807.
Isakeit, T. 2010. Pecan scab: understanding fungicide activity to prevent fungicide resistance.
Latham, A. J., and Goff, W. D. 1991. Pecan scab: A review and control strategies. Pages 89-93 in Pecan Husbandry: Challenges and Opportunities. First National Pecan Workshop Proceedings. Unicoi State Park, Georgia, July 23-24, 1990.
Latham, A. J., and Rushing, A. E. 1988. Development of Cladosporium caryigenum in pecan leaves. Phytopathology 78:1104-1108.
Lee, J., Mulder, P., and Driever, G. 2013. Commercial Pecan Insect and Disease Control. Oklahoma Cooperative Extension Service. Bulletin CR-6209.
Littrell, R. H., and Bertrand, P. F. 1981. Management of pecan fruit and foliar diseases with fungicides. Plant Disease 65: 769-774.
National List of Allowed and Prohibited Substances. 2000. Code of Federal Regulations, title 7, §205.601. Synthetic substances allowed for use in organic crop production.
Nolen, R. E. 1926. Pecan scab. Bulletin 181 of the University of Florida Agricultural Experiment Station. 26 pp.
Schubert, K., Ritschel, A., and Braun, U. 2003. A monograph of Fusicladium s. lat. (Hyphomycetes). Schlechtendalia 9:1-132.
Smith, G.S., M.H. O'Day, and W. Reid. 1995. Pecan Pest Management: Insects and Diseases. Bulletin MP711 of the University of Missouri Extension.
Stevenson, K. 1999. Fungicide resistance management in pecans. In: McCraw, B.E., E. H. Dean, and B. W. Woods, eds. The Pecan Industry: Current Situation and Future Challenges, Third National Pecan Workshop Proceedings, USDA Agricultural Research Service, 1998-04. USA: USDA, pages 1-6.
Turechek, W.W. and K. L. Stevenson. 1998. Effects of host resistance, temperature, leaf wetness, and leaf age on infection and lesion development of pecan scab. Phytopathology 88:1294-1301.
University of Georgia College of Agricultural and Environmental Sciences. Undated website. Pecan Breeding: Cultivar Information. Review of Scab Resistant Cultivars.
Vann, S. Undated. Home Pecan Diseases and Control. University of Arkansas Division of Agriculture Research and Extension. Technical bulletin, FSA7540.
