Paul Vincelli
University of Kentucky
Vincelli, P. 2002. QoI (Strobilurin) Fungicides: Benefits and Risks. The Plant Health Instructor. DOI: 10.1094/PHI-I-2002-0809-02. Updated, 2012.
Introduction
Many of the newest and most important disease-control chemicals are in the QoI family of fungicides. The first fungicides in this family were isolated from wood-rotting mushroom fungi, including one called Strobilurus tenacellus. The name strobilurin was coined for this chemical family of fungicides in recognition of the source of the first compounds of this type. (These fungicides are now more properly referred to as QoI fungicides, which is explained in the section on fungicide resistance.) These natural fungicides were thought to help the fungus defend itself from competition by microbes present in rotting wood.
Industry chemists improved on these natural fungicides by making chemical modifications that resulted in compounds which were less subject to breakdown on the leaf surface by sunlight. Several of the QoI fungicides currently registered in the United States are considered by the Environmental Protection Agency to be reduced-risk pesticides. This means these compounds pose less risk to human health and/or the environment than alternative pesticides available at the time of their commercial introduction.
Spectrum of Activity
With important exceptions, the QoI fungicides control an unusually wide array of fungal diseases, including diseases caused by water molds, downy mildews, powdery mildews, leaf spotting and blighting fungi, fruit rotters, and rusts. They are used on a wide variety of crops, including cereals, field crops, fruits, tree nuts, vegetables, turfgrasses, and ornamentals.
While QoI fungicides provide important benefits, like all fungicides, their use as replacements for older fungicides can sometimes led to unexpected changes in disease activity. For example, in turfgrasses, azoxystrobin provides excellent control of a number of important diseases. However, it can sometimes enhance the severity of certain diseases, such as dollar spot of creeping bentgrass and Pythium blight of tall fescue. Mechanisms of disease enhancement are not understood, but one possibility is that use of azoxystrobin at labeled field rates may suppress certain naturally occurring microorganisms that are antagonistic to the pathogen.
|
Trade names, examples |
Active Ingredient |
Manufacturer/Marketer |
|
Abound™ 2.08F, Amistar™, Heritage™, Quadris™ |
azoxystrobin |
Syngenta |
|
Reason™ 500SC |
fenamidone |
Bayer |
|
Disarm™ 480SC, Evito™ 480SC |
fluoxastrobin |
Arysta |
|
Sovran™ 50WG |
kresoxim methyl |
Cheminova |
|
Cabrio™ 20EG, Headline™ 2.08EC, Insignia™ 20WG, |
pyraclostrobin |
BASF |
|
Compass™ 50WG, Flint™ 50WG, Gem™ 500SC |
trifloxystrobin |
Bayer |
Table 1. QoI fungicides commercially available or expected to be available soon in the U.S. (Updated 2012)
Mobility
All of the QoI fungicides listed in Table 1 exhibit translaminar movement (which means "across the lamina", or leaf blade). When these fungicides are applied, most of the active ingredient is initially held on or within the waxy cuticle of plant surfaces (for example, see Figure 1). Some of the active ingredient "leaks" into the underlying plant cells. For those fungicides with an affinity for the waxy cuticle (such as trifloxystrobin and kresoxim methyl), active ingredient that "leaks" all the way through the lamina quickly rebinds to the cuticle on the far side of the leaf blade. Thus, the fungicide can be found on both leaf surfaces even if only one leaf surface was treated. Translaminar movement can take one to several days to be fully effective.
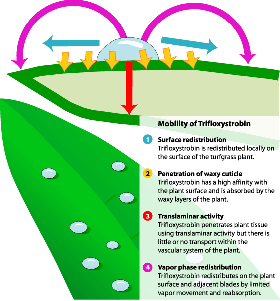
Figure 1. Mobility of trifloxystrobin, an example of a QoI fungicide. |
The fungicide azoxystrobin moves translaminarly as well as systemically (in the plant's vascular system, or "plumbing"). The fungicides kresoxim methyl and trifloxystrobin move translaminarly but not systemically. These latter fungicides, however, appear to move as a gas in the layer of still air adjacent to the leaf surface called the boundary layer. As they move in the vapor phase, they readily re-bind to the cuticle (Fig. 1). Fungicides such as kresoxim methyl and trifloxystrobin--which are not true systemics but which redistribute by these other mechanisms--have been referred to as "mesostemics", "quasi-systemics", or "surface systemics".
In terms of practical significance, systemic movement (when it occurs) and translaminar movement help to compensate for incomplete spray coverage. Redistribution in the vapor phase can also help compensate for poor crop coverage, but only to a limited extent. These processes may be especially important in crops with dense or difficult-to-spray canopies (cucurbits, for example). Be aware that several days may be required for adequate protection to be achieved via translaminar movement. Thus, growers may not achieve optimum disease control if a QoI fungicide is applied with incomplete coverage within 24 hr of an infection period.
Another practical consequence of the dynamics of translaminar movement concerns curative disease control. QoI fungicides are excellent as preventive fungicides, because they all effectively kill germinating spores. However, several of them provide poor performance against certain diseases when used curatively--that is, after infection has taken place. Recall that some QoI fungicides bind tightly to the cuticle, where most of the active ingredient can be found. Even though the active ingredient "leaks" into the leaf blade, it has such a strong affinity for the cuticle that it quickly re-binds with it when the chemical reaches the other side of the leaf. Consequently, at any one time, the dose of active ingredient actually present inside the leaf blade may be low, sometimes too low to suppress the growth of fungi within the leaf. Furthermore, for a number of fungal pathogens, the germinating spore (which starts the infection process on the outside of the plant) is more sensitive to QoI fungicides than is the mycelium (the fungal life stage found inside the plant). Thus, the best use of QoI fungicides is to apply them before infection takes place.
Effects on Plant Health Independent of Disease Control
Growth enhancement. Several QoI fungicides are known to cause growth-promoting effects in certain plants. For example, kresoxim methyl has been shown to cause changes in the hormonal balance of wheat which results in increased grain yield, apparently from delayed leaf senescence and water-conserving effects. Growth-enhancing effects independent of disease control have been observed in QoI-treated plants of several species, although these effects are very much dependent on the crop, the fungicide used, and environmental conditions.
Phytotoxicity. While the QoI fungicides are very valuable for disease control, several are known to cause phytotoxicity in certain, limited circumstances; these are described in product labels. For example, apple cultivars with a genetic background which includes MacIntosh are extremely sensitive to azoxystrobin. Indeed, these varieties are so sensitive that they can be injured when a sprayer is used to apply azoxystrobin to another crop (grapes, for example), rinsed, and then used to apply another fungicide to the apple crop! Another example: while trifloxystrobin may be used safely on most grapes, it can cause injury to Concord grapes. And kresoxim methyl is phytotoxic to certain sweet cherry varieties but not others. Producers should be aware of phytotoxicity concerns both for the treated crop and because of the possibility of injury via spray drift.
Another aspect of the phytotoxicity risk is the possibility that tank-mixes of QoI fungicides with materials that solubilize the cuticle-oils, surfactants, certain liquid formulations of insecticides-could increase their phytotoxicity potential. Although some of the active ingredient can be found within the host tissue, much of the dose of QoI fungicides remains on or within the plant cuticle. Application of a spray material that causes abnormally high levels of these fungicides to penetrate into the host tissue could potentially cause phytotoxicity on certain crops or varieties where none is normally expected. Obviously, when applying a previously unused tank-mix on a particular crop variety, a wise practice is to test-apply to small areas before treating large acreages.
Resistance
All QoI fungicides share a common biochemical mode of action: they all interfere with energy production in the fungal cell. To be precise, they block electron transfer at the site of quinol oxidation (the Qo site) in the cytochrome bc1 complex, thus preventing ATP formation. The preceding sentence may "seem like Greek" to even the most knowledgeable crop consultant, but it contains an important point-that the mode of action of the QoI fungicides is highly specific. Of the millions of biochemical reactions that occur in the fungal cell, these fungicides interfere with just one, very specific biochemical site. It is a very important biochemical site for the fungus, to be sure, but it is just one site. Thus, these are called site-specific fungicides. This is important because, commonly, just one mutation at that biochemical site (the target site of the fungicide) can result in a fungicide-resistant strain. If such a fungicide-resistant strain occurs, repeated application of QoI fungicides can lead to buildup of a fungicide-resistant pathogen subpopulation.
Experience with the QoI fungicides worldwide indicates there is a high risk of development of resistant pathogen subpopulations. Worldwide, resistance has been reported in an increasing number of pathogens of field crops, fruit, vegetable, and nut crops, ornamentals and turfgrass.
There are two general types of fungicide resistance: quantitative and qualitative. With quantitative resistance, resistant strains are somewhat less sensitive to the fungicide as compared to the wild type, but they often can still be controlled with higher rates and/or more frequent applications (within labeled limits, of course). A good example of this type of resistance is that observed with strains resistant to the DMI (demethylation-inhibitor) fungicides, such as propiconazole or triadimefon. With qualitative resistance, the resistant strain is vastly less sensitive to the active ingredient, and is no longer controlled at labeled field rates. The effect on disease control is the same as if one were spraying water on the crop instead of a fungicide. A good example of this type of resistance is that observed with the benzimidazole fungicides, such as benomyl or thiophanate methyl. Natural occurrences of resistance to the QoI fungicides indicate that most cases of control failure are due to resistance of the qualitative type, but that instances of quantitative resistance to certain QoI fungicides have also been recorded.
Fungicides that share a common biochemical mode of action for poisoning the fungus are thought to be in the same "fungicide family" and are assigned a FRAC code unique for that group. (FRAC stands for "Fungicide Resistance Action Committee". FRAC is an organization composed of scientists from manufactures of the various at-risk fungicides.) When different fungicidal products share a common mode of action, the fungus does not distinguish between the fungicides, even if the chemical structure of the active ingredients is different and the fungicides are produced by different manufacturers. Biochemically, the fungus sees them all as the same active ingredient. When a fungus is resistant to one fungicide in a chemical family, it is usually resistant to all fungicides in that family. This is called cross resistance. In many situations, fungal strains resistant to QoI fungicides exhibit cross-resistance to other QoI fungicides. In such cases, efficacy of all QoI fungicides may be compromised, even if some of them have never been used on that farm. Cross-resistance only applies within a given chemical family. Therefore, QoI-resistant subpopulations can be controlled with other fungicides not in the QoI family.
Guidelines for Reducing Resistance Risk
Start by understanding that there is nothing a grower can do that will eliminate the risk of fungicide resistance, except to never use the at-risk fungicide. One can reduce the risk of its development by following practices that delay development of a resistant subpopulation.
Guidelines for reducing the risk of resistance against fungicides are issued by the FRAC. FRAC is an organization composed of scientists from manufacturers of the various at-risk fungicides. The guidelines issued by FRAC for each fungicide family are based on scientific principles and up-to-date research. Thus, FRAC guidelines provide a basis for understanding how to reduce the risk in the cropping situations where you work.
In addition to the key guidelines described below, growers should understand that reducing the risk of fungicide resistance begins by using non-fungicidal means for disease control: crop rotation, selection of varieties with reduced susceptibility, sanitation, pathogen-free seed, etc. These practices help reduce overall disease pressure. The occurrence of an adapted mutant with resistance to a fungicide is a matter of chance, like a "roll of the dice". The larger the pathogen population, the greater the chance that such a mutant will arise. Reducing disease pressure through non-chemical practices helps lower the chance that a fungicide-resistant mutant will occur; it does this by keeping the overall size of the pathogen population small
Limit the number of applications of QoI fungicides (=FRAC Group 11 fungicides) in a given season. The basis of this guideline is this: the more often a QoI fungicide is used, the higher is the selection pressure towards the development of a resistant subpopulation. Limiting the number of applications reduces the opportunity for selection pressure, potentially extending the useful life of the QoI family of fungicides on a particular crop. This guideline is indicated on product labels, and label restrictions on the seasonal total number of sprays apply to all QoI fungicides, not just to the product being used. For example, as of the date of publication, the label for Flint 50WG™ instructed the apple grower not to apply more than four sprays of Flint 50WG™ or other strobilurin (=QoI fungicides) during the same season. If a grower makes two applications of Flint 50WG™ in a given season, only two more applications of any QoI fungicide could be made that season, whether it be Flint 50WG™ or Sovran 50WG™.
The seasonal limit is higher on crops that receive 8-12 sprays per year than those that receive less. Obviously, if the seasonal limit for QoI fungicides is reached and the crop still needs protection against disease for a longer period of time, alternative fungicides must be used. These alternatives must, of course, not be in the QoI group and must be from other fungicide families. Under the current FRAC guidelines, seed treatments and in-furrow treatments do not count towards the seasonal limit, because of the limited mobility of these fungicides within plants.
Limit the number of consecutive applications of a QoI fungicide. The product labels indicate the number of consecutive applications of QoI fungicides that are allowed on each crop, before the user must switch to an equal number of applications of non-QoI fungicides. For most crops, the number of consecutive applications will be limited to two before the grower must switch to a fungicide with a different mode of action. FRAC guidelines on certain crops are even more strict; for example, on cucurbits, it is advised never to apply QoI fungicides consecutively. Like the seasonal limit described above, this guideline is designed to reduce the opportunity for selection pressure towards resistance.
Mixing QoI fungicides with other fungicides can reduce selection pressure towards resistance. Mixtures do not prevent resistant mutants from arising on a farm. They can, however, can slow the rate of spread of these mutants. A proper mixing partner is one that provides satisfactory disease control when used alone on the target disease. Also, the mixing partner must be from some fungicide family other than the QoI group. Tank-mixing fungicides from the same chemical family does nothing to reduce the risk of fungicide resistance. The application rates of the components should not be reduced below the minimal labeled rate.
From the standpoint of fungicide resistance, wisest use of QoI fungicides may be to use them at the early stages of disease development. Some researchers believe that curative use of a fungicide increases the risk of resistance, because the producer is treating a much larger population of spores and mycelium (the body of the fungus) than would be treated preventively. Allowing a buildup of a large population of spores before treatment increases the chances that a resistant mutant will be present when the chemical is applied.
Additional guidelines. While translaminar movement is a wonderful feature of the QoI fungicides, potential problems arise when a QoI fungicide is tank-mixed with a contact (=protectant) fungicide for resistance-management purposes. Since contact fungicides remain on the treated leaf surface, poor coverage of the underside of crop foliage could result in biologically active levels of QoI fungicides there through translaminar movement without the presence of the mixing partner. On such leaf surfaces, a QoI-resistant strain could take hold and flourish, should it arise. Whenever tank-mixing a QoI fungicide with a contact fungicide, always strive for complete coverage of all plant surfaces.
As noted above, growth-enhancing effects independent of disease control have been observed in QoI-treated plants of several species. Where these cases occur, this could pose an incentive to use a product inappropriately or excessively. While optimizing plant health is always an important objective, inappropriate use or overuse use of a QoI fungicide for its growth-promoting qualities could be a violation of the product label, and it may also increase selection pressure towards fungicide resistance. Users should be very mindful not to overuse any at-risk fungicide. Once resistance to QoI fungicides develops on a farm, there is a very good chance that efficacy of these products against that disease will be compromised for quite some time.
Acknowledgments
Thanks to Hendrik Ypema and Gilberto Olaya for reviewing and providing helpful comments on a previous draft of the article. Also, appreciation is expressed to G. Olaya, John Smith, and Kyle Miller for assistance in updating Table 1.
