Sherwood, J.L., German, T.L., Moyer, J.W. and D.E. Ullman. 2003. Tomato spotted wilt. The Plant Health Instructor. DOI:10.1094/PHI-I-2003-0613-02
Updated, 2009
Tomato spotted wilt
Tomato spotted wilt virus
Over 1000 species in over 85 families, including many vegetables, peanut and tobacco.
Authors
John L. Sherwood, University of Georgia
Thomas L. German, University of Wisconsin-Madison
James W. Moyer, North Carolina State University
Diane E. Ullman, University of California-Davis

Figure 1 |

Figure 2 |
|
The "spotted wilt" disease of tomato was first described in Australia in 1915 and was determined to be of viral etiology in 1930 (Figs. 1,2). The Tomato spotted wilt virus (genus Tospovirus; family Bunyaviridae) (TSWV) was thought to be the sole member of the tomato spotted wilt virus group until 1989 when Impatiens necrotic spot virus was characterized. The genus Tospovirus now contains TSWV as the type member and more than a dozen other distinct viruses whose identification has been facilitated by the use of serological and molecular techniques. The tospoviruses are transmitted by thrips (Thysanoptera: Thripidae) and replicate in both the thrips vectors and the plant hosts. There are significant differences in tospoviruses and their relationship with specific thrips species, so what is presented herein on TSWV is not applicable to all tospovirus-thrips interactions.
Symptoms and Signs
TSWV infects over 1000 plant species and causes significant economic damage to many agronomic and horticultural corps. In some areas the virus has been found to be ubiquitous in the environment as it can infect many weeds, landscape plants, and native plants. Symptoms of tomato spotted wilt differ among hosts and can be variable in a single host species. Stunting is a common symptom of TSWV infection, and is generally more severe when young plants are infected (Figure 3). Chlorotic or necrotic rings form on the leaves of many infected hosts (Figure 4), and may also appear on the fruits of some hosts (Figure 1). Necrosis may develop in the foliage of some hosts, making diagnosis based on symptoms alone difficult (Figure 5). Although TSWV is not seed transmitted, it may cause the discoloration of seed produced on infected hosts (Figure 6). Hence, tomato spotted wilt may affect both the quantity and quality of plant products. Additionally, movement of infected plant material may result in TSWV being introduced into a new setting.

Figure 3 |

Figure 4 |

Figure 1 |

Figure 5 |

Figure 6 |
Pathogen Biology
Virions of TSWV are complex compared to many plant viruses. There are three RNAs in the virus genome that are individually encapsidated, and are collectively bound by a membrane envelope that is of host origin (Figure 7). This complex virion structure is a characteristic that distinguishes TSWV from most other plant viruses. TSWV virions are roughly spherical and are 80-110 nm in diameter (Figure 8). Two virus proteins processed during replication to contain sugars, i.e. glycoproteins (GPs), are dispersed throughout the surface of the viral envelope. These proteins are called glycoprotein C (GC) and glycoprotein N (GN) and differ slightly in size (Figure 7). Inside the viral envelope is each of the three viral RNAs, individually bound by multiple copies of a nucleocapsid protein. The three RNAs differ in size and are called Large (L), Middle (M) and Small (S) (Figure 9). Also inside the envelope are several copies of a virus-encoded "replicase" protein that is required to initiate virus replication in a new host.
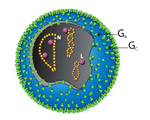
Figure 7
|
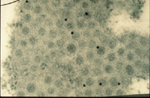
Figure 8 |
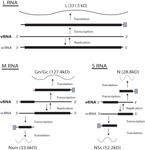
Figure 9 |
The GPs in the envelope function in the maturation and assembly of virions, and appear to play a role in the acquisition of TSWV by thrips. Envelope-deficient isolates of TSWV, generated by serial mechanical passage in plants, are infectious in plants but are not transmitted by thrips. This is evidence that the GPs are not required for replication in plants, but are required for virus infection of thrips leading to subsequent virus replication in and transmission by thrips.
In addition to the GPs, the M RNA segment encodes a non-structural protein (NSm). The NSm is unique to tospoviruses in the family Bunyaviridae, and is thought to be an adaptation of tospoviruses to plants to facilitate tospovirus movement from cell to cell through plant cell walls via the plasmodesmata. Because enveloped particles are too large to be transported through plasmodesmata, the role of NSm is to form tubules that facilitate movement of nucleocapsids (RNA plus protein) from cell to cell (Figure 10). In addition to the nucleocapsid protein, the S RNA segment encodes a non-structural protein (NSs). Crystalline-like structures of NSs are produced in infected insect cells (Figure 11) and plant cells. The NSs protein has RNA silencing suppressor activity, and may play a role in post-transcriptional gene silencing or RNA metabolism.
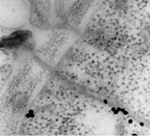
Figure 10 |

Figure 11 |
TSWV is one member of the dozen or so different viruses in the genus Tospovirus. One striking difference in these viruses is the variation in their host ranges. TSWV is renowned for having an extensive host range, whereas other members of the genus Tospovirus such as Peanut yellow spot virus or Iris yellow spot virus have narrow host ranges. The genetic diversity in this virus group may be fostered by their replication in both different species of plants and different species of thrips. Although genes to confer resistance to TSWV have been found in some germplasm lines and used to develop new cultivars, there has been rapid adaptation of new forms of the virus to cultivars that have been released. Thus, there are virtually no cultivars of major crops with significant levels of resistance to TSWV that have remained resistant in the field for more than a few years.
Disease Cycle and Epidemiology
Thrips deposit their eggs into plant tissue and the eggs hatch after 2-3 days, depending on the temperature and the plant host species (Figure 12). There are two feeding larval stages that are followed by two non-feeding pupal stages. The life cycle takes about 20-30 days from egg to adult, again depending on the temperature. Thrips disperse over long distances by wind. TSWV must be acquired by thrips during the larval stage of their development to be transmitted. Thus, only immature thrips that acquire TSWV, or adults derived from such immatures, transmit the virus. The ability of thrips to acquire TSWV decreases as the thrips age. Although the time in development that thrips can acquire the virus is limited, the wide host range for both virus and thrips facilitates development of epidemics. Once acquired by the larvae, the virus is passed transtadially, i.e. TSWV persists through insect molts from larval to adult stages. The virus replicates in thrips, and the thrips can transmit the virus during their entire life. There are at least ten species of thrips that transmit tospoviruses. Frankliniella occidentalis, the western flower thrips (WFT) (Figure 13), is considered to be the most important vector species because it is globally distributed and can transmit most tospoviruses.
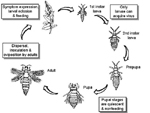
Figure 12 |

Figure 13 |
The acquisition of TSWV by thrips larvae is an area of continuing study. Some evidence indicates that the viral glycoproteins (GPs) bind to the midgut epithelium and have a role in the process of virus uptake in the midgut (Figure 14). The virus then moves to other cells and organs, and becomes well established in the muscle cells (Figure 15). Another perspective is that the temporary association between the midgut, visceral muscle and salivary gland complex in the larval stage provides the avenue for the virus to become systemically established in the thrips. Eventually, the virus enters the salivary glands. Although the route to the salivary glands still needs to be determined, virions are excreted with the saliva (Figure 16) into host plants during thrips feeding.
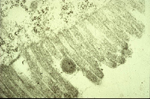
Figure 14 |
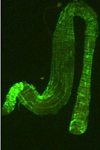
Figure 15 |
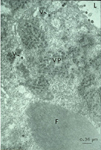
Figure 16 |
The epidemiology of the diseases caused by TSWV remains poorly understood. It was only in 1993 that it was confirmed that TSWV multiplies in its vector. The wide host range of TSWV and the occurrence of at least ten species of thrips that transmit TSWV make eliminating the sources of primary inoculum of the virus impractical. Additionally, viruliferous thrips may overwinter in the field. The population of viruliferous thrips and the stage of crop development appear to be crucial in disease development. For lettuce grown in Hawaii, it was found that the earlier the crop is infected with the virus, the more severe the loss. An elegant model developed to demonstrate the potential economic impact of TSWV in lettuce helped growers determine if they should continue to grow the crop once virus infection was confirmed.
Disease Management
The usual approaches to management of diseases caused by viruses are host resistance, vector control or avoidance. While natural plant resistance to TSWV and other tospoviruses has been identified in a number of agronomic and horticultural crops, virus isolates capable of overcoming host resistance have readily appeared. Considerable effort has been expended to develop transgenic plants that have virus-derived genes to confer resistance to tospovirus infection. Transgenic tospovirus-resistant plant species that have been developed include chrysanthemum, peanut, tobacco, and tomato (Figure 17). To date, these have not been used commercially.

Figure 17 |
Use of insecticides alone to control thrips populations in the field is often ineffective. Contact insecticides generally do not reach where the thrips are located on the plant, and systemic insecticides do not act rapidly enough to prevent virus transmission. As more is learned about thrips feeding, treatments that deter feeding or induce host resistance mechanisms to deter thrips feeding may be used. Some success in this approach has been achieved in lettuce. Reflective mulches placed over plant beds prior to planting can disorient thrips or impair thrips feeding. Mulches have shown some utility in disease management, but results have not been consistent between different crops. Although elimination of thrips in the field is not practical, it is possible to reduce thrips populations in greenhouses. Screening air intakes and using double-door entries have helped reduce incidence of TSWV by preventing the entry of viruliferous vectors into the greenhouse. While avoidance or exclusion of thrips may be feasible in a greenhouse, it is generally not viable as a sole management practice in the field because of the wide host range of TSWV and the number of thrips species that transmit the virus. However, in some areas timing of planting to avoid major thrips migrations during critical early plant growth periods is feasible to reduce disease.
An integrated approach that addresses many parameters that affect tomato spotted wilt development has been successful in mitigating tomato spotted wilt in peanut in the Southeastern United States. Peanut variety, planting date, plant population, insecticide application, disease history, row pattern and tillage were identified as factors affecting disease development. These factors are weighted to determine a "risk index" for TSWV in the crop. The grower obtains a low, moderate or high risk value that can be considered when implementing crop production practices (Figure 18; https://tswv.caes.uga.edu/peanut.html).

Figure 18 |
Indicator plants have been useful as an early warning system for the presence of infective thrips in certain ornamental crops. This strategy was most useful in identifying significant sources of infective thrips that could be removed as a preventive measure. In one California cut flower operation, the identification and removal of TSWV sources using indicator plants resulted in a reduction of virus incidence from 74% to 1%. Thus, crop specific integrated approaches for spotted wilt management will likely have to be developed. Continuing to elucidate the epidemiology of TSWV and to develop and deploy crops with natural and genetically engineered resistance may result in more effective management of tomato spotted wilt in the future.
Selected References
Best, R.J. 1968. Tomato spotted wilt virus. Advances in Virus Research 13:66-146.
Mound, L.A. 2005. Thysanoptera: Diversity and Interactions. Annual Review of Entomology 50:247-269.
Moritz, G. http://www.biologie.uni-halle.de/org/thripsnet/
Moritz, G., S. Kumm, and L. Mound. 2004. Tospovirus transmission depends on thrips ontogeny. Virus Research 100:143-149.
Sin, S-H., B.C. McNulty, G.G. Kennedy, and J.W. Moyer. 2005. Viral genetic determinants for thrips transmission of Tomato spotted wilt virus. Proceedings of the National Academy of Sciences 102:5168-5173.
Takeda, A., K. Sugiyama, H. Nahgano, M. Mori, M. Kaido, K. Mise, S. Tsuda, and T. Okuno. 2002. Identification of a novel RNA silencing suppressor, NSs protein of Tomato spotted wilt virus. Federation of European Biochemical Societies (FEBS) Letters 532:75-79.
The Complete Tospovirus Resource Page http://www.oznet.ksu.edu/tospovirus/
Thrips Biology and Management http://thrips.ifas.ufl.edu/
Ullman, D.E., R. Meideros, L.R. Campbell, A.E. Whitfield, J.L. Sherwood and T.L. German. 2002. Thrips as vectors of tospoviruses. Advances in Botanical Research 36:113-140.
Virus databases on-line http://image.fs.uidaho.edu/vide/genus049.htm
Whitfield, A.E., K.K.N. Kumar, D. Rotenberg, D.E. Ullman, E.A. Wyman, C. Zietlow, D.K. Willis, and T.L. German. 2008. A soluble form of the tomato spotted wilt virus (TSWV) glycoprotein GN (GN-S) inhibits transmission of TSWV by Frankliniella occidentalis. Phytopathology 98:45-50.
Whitfield, A.E., D.E. Ullman, and T.L. German. 2005. Tospovirus-Thrips Interactions. 2005. Annual Review of Phytopathology 43:459-489.
Yudin, L.S., B.E. Tabashnik, J.J. Cho and W.C. Mitchell. 1990. Disease prediction and economic models for managing tomato spotted wilt virus diseases in lettuce. Plant Disease 74:211-215.
