Scholthof, K-B.G. 2000. Tobacco mosaic virus. The Plant Health Instructor. DOI: 10.1094/PHI-I-2000-1010-01
Updated 2005.
Tobacco mosaic
Tobacco mosaic virus
Tobacco, tomato, and other solanaceous plants
Author
Karen-Beth G. Scholthof
Texas A&M University

Typical mosaic pattern on flue-cured tobacco leaves
infected with Tobacco mosaic virus. |
TMV was the first virus to be discovered over a century ago and was the first virus ever purified. It has since yielded fascinating insights into how viruses infect their hosts. Research on TMV has also led to major Nobel prize winning discoveries on general principles of life.
Symptoms and Signs
Symptoms induced by Tobacco mosaic virus (TMV) are somewhat dependent on the host plant and can include mosaic, mottling (Figures 1 and 2), necrosis (Figures 3 and 4), stunting, leaf curling, and yellowing of plant tissues. The symptoms are very dependent on the age of the infected plant, the environmental conditions, the virus strain, and the genetic background of the host plant. Strains of TMV also infect tomato, sometime causing poor yield or distorted fruits, delayed fruit ripening, and nonuniform fruit color (Figure 5).

Figure 1 |

Figure 2 |

Figure 3 |

Figure 4 |

Figure 5 |
Pathogen Biology
Hosts for TMV include tobacco (Figure 1), tomato (Figure 5), and other solanaceous plants. Currently, yield losses for tobacco due to TMV are estimated at only 1% because resistant varieties are routinely grown. In contrast, losses of up to 20% have been reported for tomato. In addition, poor fruit quality may reduce the value of the crop on the commercial fresh market.

Figure 1 |

Figure 5 |
TMV is the type member of a large group of viruses within the genus Tobamovirus. The rod-shaped virus particles (virions) of TMV measure about 300 nm x 15 nm (Figure 6). A single TMV particle is composed of 2,130 copies of the coat protein (CP) that envelope the RNA molecule of about 6,400 nucleotides (Figure 7). This single-stranded RNA encodes four genes: two replicase-associated proteins that are directly translated from the TMV RNA, and the movement protein and a coat protein that are translated from subgenomic RNAs (Figures 8 and 9).
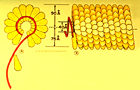
Figure 7 |
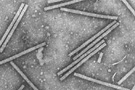
Figure 6 |
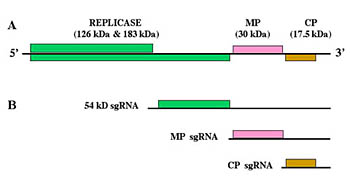
Figure 8 |
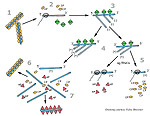
Figure 9 |
Disease Cycle and Epidemiology
Transmission from plant to plant
TMV is very easily transmitted when an infected leaf rubs against a leaf of a healthy plant, by contaminated tools, and occasionally by workers whose hands become contaminated with TMV after smoking cigarettes. A wounded plant cell provides a site of entry for TMV. The virus can also contaminate seed coats, and the plants germinating from these seeds can become infected. TMV is extraordinarily stable. Purified TMV (Figure 6) has been reported to be infectious after 50 years storage in the laboratory at 4°C/40°F.

Figure 6 |
Replication
TMV enters the plant cell through minor wounds. Once TMV enters the cell, the virus particles disassemble in an organized manner to expose the TMV RNA. The virus RNA is positive-sense, or "+ sense", and serves directly as a messenger RNA (mRNA) that is translated using host ribosomes. Translation of the replicase-associated proteins (RP) 126- and 183-kDa) begins within a few minutes of infection.
As soon as these proteins have been synthesized, the replicase associates with the 3' end of the + sense TMV RNA for the production of a negative sense, or "- sense", RNA. The - sense RNA is the template to produce both full-length genomic + sense RNA as well as the + sense subgenomic RNAs (sgRNAs) (Figure 8)

Figure 8 |
The sgRNAs are translated by the host ribosomes to produce the movement protein (MP) (30 kDa) and the coat protein (CP) (17.5 kDa). The coat protein then interacts with the newly synthesized + sense TMV RNA for assembly of progeny virions.
These virus particles are very stable and, at some point when the cells are broken or the leaf dries up, they are released to infect new plants. Alternatively, the + sense TMV RNA is wrapped in movement protein, and this complex can infect adjacent cells.
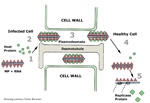
Figure 10 |
Movement in the infected plant
TMV uses its movement protein to spread from cell-to-cell through plasmodesmata, which connect plant cells (figure 10). Normally, the plasmodesmata are too small for passage of intact TMV particles.
The movement protein (probably with the assistance of as yet unidentified host proteins) enlarges the plasmodesmatal openings so that TMV RNA can move to the adjacent cells, release the movement protein and host proteins, and initiate a new round of infection. As the virus moves from cell to cell, it eventually reaches the plant's vascular system (veins) for rapid systemic spread through the phloem to the roots and tips of the growing plant.
Epidemiology
The TMV disease cycle and its epidemiology are intimately related because the virus is completely dependent on the host for replication and spread. There is wide variation in disease incidence, depending on the time of disease onset in the field and on cropping practices. For example, a few plants could become infected early in the season, either from TMV on the seed coat or by workers contaminating plants. The disease could then spread rapidly throughout the field or greenhouse by TMV-infected plants contacting healthy plants, or by equipment or workers. TMV can also survive or overwinter in infected plant debris or perennial (weedy) hosts and, perhaps, in the soil. Agricultural practices, such as continuous cropping, have the potential to be a particular problem, especially in greenhouse facilities, where TMV inoculum may increase in more than one plant species.
Disease Management
Greenhouse management
Horticultural practices. To reduce infection of plants with TMV all tools should be washed with soap or a 10% solution of household bleach to inactivate the virus. TMV-contaminated soil should be discarded. To avoid transmitting the virus from an infected plant to healthy plants, the watering hose or watering can should not be allowed to make contact with the plants. Care should be taken to dispose of dead leaves and old plants, because dry, TMV-infected leaves can be blown around the greenhouse as 'dust' which can subsequently infect healthy plants if they are wounded.
Cross protection. Inoculation of a mild strain of the virus onto young plants can protect them from subsequent infection by more severe strains of TMV. This is a well documented control strategy, called "cross protection," that is successfully applied in greenhouse operations. Transgenic plants also offer alternative strategies for virus control (see Biotechnology) (Figure 11).

Figure 11 |
Preplanting options (greenhouse and field)
Cultivars. Several tobacco and tomato cultivars have been bred to be genetically resistant to TMV.
Biotechnology. Genetic engineering techniques have provided scientists with the ability to express the TMV coat protein gene in transgenic tobacco and tomato plants. This control strategy can safeguard the plants from infection by closely related strains of the virus (Figure 11).
Elimination of inoculum. Under experimental conditions, it has been shown that TMV can be inactivated when workers dip their contaminated hands in milk prior to planting. This inexpensive technique greatly reduces the incidence of disease (Figure 12). Seedlings that are known to be susceptible should not be transplanted into soil that contains TMV-contaminated root or plant debris.

Figure 12 |
Management in the field
Scouting for disease. During the growing season, infected plants should be dug up, bagged, and removed from the field. Rotation practices that include resistant plants or non host crops also should be employed to reduce the amount of inoculum in the field.
Management at harvest and in storage
TMV can easily overwinter on the seed coat, thus providing an inoculum source for the next planting cycle. Therefore, it is important to treat TMV-contaminated tobacco seed with a 10% solution of trisodium phosphate for 15 minutes. Alternatively, tomato seed contaminated with TMV can be incubated at 70°C/158°F for 2-4 days prior to planting. Both treatments will inactivate the virus that is on the seed coat, but should have little negative effect on seed germination.
Significance
In 1898, Martinus W. Beijerinck, of the Netherlands, put forth his concepts that TMV was small and infectious. Furthermore, he showed that TMV could not be cultured, except in living, growing plants. This report, suggesting that 'microbes' need not be cellular, was to forever change the definition of pathogens. In 1946, Wendall Stanley was awarded the Nobel Prize for his isolation of TMV crystals, which he incorrectly suggested were composed entirely of protein. Research by F.C. Bawden and N. Pirie, in England, during the same period correctly demonstrated that TMV was actually a ribonucleoprotein, composed of RNA and a coat protein. By the mid-1950s, scientists in Germany and the United States proved that the RNA alone was infectious. This discovery ushered in the modern era of molecular virology. TMV is known for several 'firsts' in virology, including the first virus to be shown to consist of RNA and protein, the first virus characterized by X-ray crystallography to show a helical structure (Figure 7), and the first virus used for electron microscopy (Figure 6), solution electrophoresis and analytical ultracentrifugation. TMV also was the first RNA virus genome to be completely sequenced, the source of the first virus gene used to demonstrate the concept of coat protein mediated protection (Figure 11), and the first virus for which a plant virus resistance gene (the N gene) was characterized. Today, TMV is still at the forefront of research leading to new concepts in transgenic technology for virus resistance and developing the virus to act as a 'work horse' to express foreign genes in plants for production of pharmaceuticals and vaccines.

Figure 7 |

Figure 6 |

Figure 11 |
Selected References
Abel, P.P., R.S. Nelson, B. De, N. Hoffmann, S.G. Rogers, R.T. Fraley, and R.N. Beachy. 1986. Delay of disease development in transgenic plants that express the tobacco mosaic virus coat protein gene. Science 232:738-743.
Ding, B. 1998. Intercellular protein trafficking through plasmodesmata. Plant Mol. Biol. 38:279-310.
Harrison, B.D. and T.M.A. Wilson. 1999. Tobacco mosaic virus: Pioneering research for a century. Phil. Transact. Royal Soc. London B. 354: 517-685.
Jones, J.B., J.P. Jones, R.E. Stall, and T.A. Zitter. 1991. Compendium of Tomato Diseases. APS Press, St. Paul, MN.
Nelson, R.S. and A.J.E. van Bel. 1998. The mystery of virus trafficking into, through and out of vascular tissue. Prog. Bot. 59:476-533.
Scholthof, K.-B. G. 2004. Tobacco mosaic virus: A model system for plant biology. Annu. Rev. Phytopathol. 42:13-34. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&
db=pubmed&dopt=Abstract&list_uids=15283658&query_hl=5
Scholthof, K-B.G., J.G. Shaw, and M. Zaitlin. 1999. Tobacco mosaic virus: 100 years of contributions to virology. APS Press. St. Paul, MN.
Schumann, G. L. 1991. Plant Diseases: Their Biology and Social Impact. APS Press, St. Paul, MN.
Shew, H.D. and G.B. Lucas. 1991. Compendium of Tobacco Diseases. APS Press, St. Paul, MN.
