Lima, J. A. A.; Nascimento, A. K. Q.; Lima, R. C. A. and Purcifull, D. E. 2013. Papaya lethal yellowing virus. The Plant Health Instructor. 10.1094/PHI-I-2013-0123-01
Papaya lethal yellowing
Papaya lethal yellowing virus
: Papaya (Carica papaya L.)
Authors
J. Albérsio A. Lima, Federal University of Ceará, Laboratory of Plant Virology
Aline K. Q. Nascimento, Federal University of Ceará, Laboratory of Plant Virology
Roberto C.A. Lima, BioClone – Production of Cloned Fruit Crop Plantlets
D.E. Purcifull, 3106 NW First Avenue, Gainesville, FL 32607
Author e-mail: albersio@ufc.br
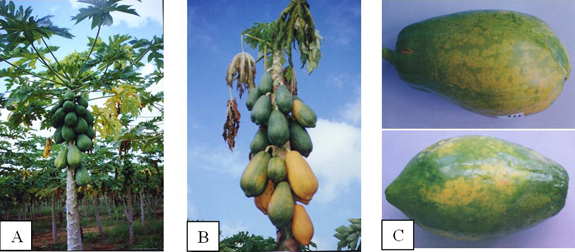
Papaya (Carica papaya) exhibiting symptoms caused by Papaya lethal yellowing virus (PLYV)
Papaya (Carica papaya) is an important tropical fruit crop and production is increasing every year in Northeastern Brazil. Papaya lethal yellowing is a disease caused by Papaya lethal yellowing virus (PLYV) that occurs only in Northeastern Brazil. The symptoms are characterized by progressive leaf yellowing and greenish circular spots on the fruits. The symptoms of inoculated young papaya plants consist of mosaic, leaf yellowing and distortion. The virus particles are isometric (30 nm), with the genome composed with a single fragment of single-stranded RNA (ssRNA) of ca. 1.6 × 106 Da and a coat protein with a single component of ca. 36 kDa. Although no biological vector has been confirmed, the virus is spreading every year, probably by infected plantlets and contaminated tools. The virus infects only C. papaya, Jacaratia heterophylla, J. spinosa, Vasconcellea cauliflora, V. monoica and V. quercifolia, all from the family Caricaceae. The virus is very stable and can be detected in dried roots and leaves maintained in laboratory conditions for up to 120 days. Phylogenetic analysis of the RNA-dependent RNA polymerase (RdRp) nucleotide sequences indicated that PLYV is a member of the genus Sobemovirus.
Symptoms and Signs
Symptoms
Symptoms begin with a progressive yellowing of leaves in the upper third of the plant canopies, which wilt and subsequently die followed by the death of the entire plant (Figure 1A, B). Greenish circular spots appear on the fruits that turn yellowish as the fruits ripen (Figure 1C). Inoculated young plants show mosaic, leaf distortion (Figure 2A) and yellowing (Figure 2B). Some studies demonstrated that the ripening process of fruits from infected plants is delayed and the pulp turns hard, which reduces the market value of the fruit, especially for exportation.
 Figure 1
Figure 1

Figure 2
Greenhouse studies with doubly-inoculated papaya plants demonstrated a clear and severe synergistic interaction between PLYV and Papaya ringspot virus (PRSV). The highly severe symptoms shown by the plants doubly infected with PLYV and PRSV consisted of chlorosis, yellowing, growth reduction, systemic necrosis and death of 50% of the inoculated plants. Doubly-infected plants with severe symptoms were also found in natural conditions. This synergistic interaction suggests that the use of a mild strains of PRSV for cross protection should not be recommended for controlling PRSV in Northern Brazil.
Host Range
Like most other virus species in the genus Sobemovirus, PLYV has a narrow host range, which is restricted to plant species from the family Caricaceae. The virus infects all commercial papaya types and varieties, and also Jacaratia heterophylla, J. spinosa, Vasconcellea cauliflora, V. monoica and V. quercifolia. The species J. heterophylla and V. monoica are not found growing naturally in Brazil but J. spinosa and V. quercifolia are cultivated in southern and western central parts of Brazil. Host range studies indicated that PLYV did not infect any of the other 82 plant species from 16 different plant families that were inoculated with the virus, including the indicator plants Chenopodium amaranticolor, C. murale, C. quinoa and Nicotina benthamiana. The absence of symptoms and virus in the inoculated plants was confirmed by indirect enzyme-linked immunosorbent assay (ELISA).
Pathogen Biology
Papaya lethal yellowing disease is caused by PLYV, which is a member of the genus Sobemovirus. PLYV particles are isometric and ca. 30 nm in diameter (Figure 3A). Large numbers of isometric virus particles can be observed by electron microscopy in cytoplasm and vacuoles of cells from leaves and fruits of infected plants (Figure 3B). The virus genome is composed of a unique fragment of single-stranded RNA (ssRNA) of ca. 1.6 × 106 Da, and its coat protein is formed by a single protein component of ca. 34.7 kDa. Highly concentrated virus was purified from infected plants; the yield was ca. 310 mg of purified virus per Kg of infected papaya leaves. Polyclonal antiserum obtained from a rabbit immunized with purified virus reacted at a dilution of 1:512,000 in indirect ELISA. Because of the high concentration of virus in infected tissues and its high immunogenicity, polyclonal antiserum specific for PLYV also was obtained by oral immunization of rabbits with extracts from infected leaves or with a purified virus preparation. PLYV is readily distinguishable serologically from PRSV and other viruses from the Potyvirus, Comovirus and Bromovirus genera.
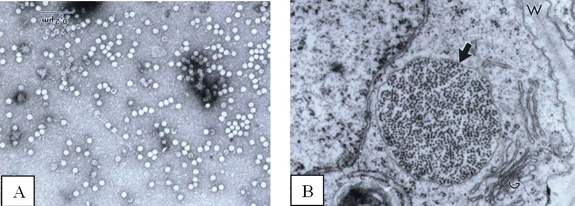
Figure 3
The virus is very stable and can be detected in dried roots and leaves maintained at laboratory conditions of approximately 26°C for up to 120 days. The virus has the following physical properties: a thermal inactivation point of 80°C, longevity in-vitro of 60 days and a dilution end point of 10-6. Nevertheless, the virus can be inactivated in leaves and roots removed from infected plants and subjected to solarization for 12 days, but maintained its infectivity when the leaves and the roots were left over the soil at natural conditions for 32 days. Alcohol and neutral detergents were not effective for inactivating PLYV on contaminated agricultural tools. However, the virus was inactivated with a 10% commercial sodium hypochlorite solution (household bleach) for five minutes.
Disease Cycle and Epidemiology
Although a natural biological vector for PLYV has not yet been identified, the virus is readily transmitted by human actions including contaminated hands, agricultural tools, soil and irrigation water. Studies indicated that the virus is not transmitted by Myzus persicae, Diabrotica bivitulla, or D. speciosa. On the other hand, infective PLYV was biologically and serologically detected in naturally contaminated soil, water that had been used to irrigate infected plants, and on the surface of seeds from infected papaya fruits. Although detected on the surface of seeds from infected fruits, the virus was not detected in the embryo of seeds nor was it transmitted by seeds harvested from infected fruits. The virus continues to spread each year. The occurrence of the virus in small patches of infected plants inside orchards or on the border of the orchards suggests that the virus is probably introduced via infected plantlets or contaminated tools and spread by grower activities from those initial sources of inocula to neighboring plants.
Disease Management
Several strategies are recommended for controlling PLYV, including the following measures: use of virus-free certified plantlets; eradication of virus-infected plants; using disinfestants to treat agricultural tools to help prevent transmission, and adoption of other cultural practices to reduce man-assisted transmission of the virus (e.g., wearing and frequently changing latex gloves). However, avoidance is the most important strategy to control PLYV.
Production of Virus-Free Plantlets
Nurseries producing papaya plantlets (Figure 4) should be isolated in areas free from virus incidence and distant from old papaya orchards that may be infected by the virus. In the past, the use of virus-infected plantlets has contributed to the spread of the virus within the production areas and states. This likely introduced the virus during orchard establishment. Infected plantlets are likely the primary source of virus within orchards and the spread of the virus in the orchards is a result of current agricultural practices. For this reason, the production of plantlets in protected nurseries far from papaya commercial production orchards represents an important control strategy for PLYV, especially when the orchards are located in a selected virus-free area. The plantlets produced should be serologically indexed and certified free of PLYV and PRSV. Agricultural companies for production of tropical fruit and horticulture plantlets located in the Northeastern Brazil are also producing papaya plantlets using good quality seeds that are free of virus (Figure 4).

Figure 4
Eradication of Sources of Virus
Virus-infected plants exhibiting symptoms should be removed from the orchards. Papaya orchards that are no longer being harvested should be eliminated even if the plants are not expressing symptoms. An efficient eradication program involves the elimination of all sources of virus in and near areas where orchards are established. The elimination of sources of PLYV in the field should be monitored by well trained technicians for recognition of virus symptoms. If virus infection is suspected, samples of suspicious plants should be serologically indexed for the presence of virus. Eradication programs should include the participation of producers and governmental institutions. In established orchards, depending on the degree of virus incidence, roguing should be practiced as a complementary control measure. Rogued plants should be submitted to solarization for a period of 15 days which has been demonstrated to be effective in inactivating the virus. The practice of roguing has been demonstrated to be efficient in several papaya-producing areas for controlling PRSV, which is efficiently transmitted by aphids. Considering the difficulties of convincing growers about the importance of eradication programs for controlling viral diseases in papaya, the Secretary of Agriculture for Sanitary Defense from the Brazilian Ministry of Agriculture approved a law that requires all papaya producers to follow the established guidelines for the eradication program for controlling papaya ringspot disease. According to the established guidelines, growers who do not follow the eradication program will have their orchards inspected and those orchards with infected plants will be condemned. The long-term success of the eradication program for controlling PRSV is questionable, especially because previous programs have lost effectiveness over time in other regions, including Hawaii. The eradication of infected plants to control PLYV would more likely be successful because PLYV does not appear to have a biological vector to spread the virus in the field.
Disinfestation of Agricultural Tools
Because of the high stability of PLYV, its inactivation on the surface of contaminated agricultural tools is considered an important control measure to avoid virus dissemination inside a particular orchard. The agricultural tools used for trimming or harvesting practices in a papaya orchard should be immersed for five minutes in a 10% solution of commercial household bleach (which contains sodium hypochlorite) after use with each plant.
Avoid Virus Transmission
Because of the high stability of PLYV, care should be taken to avoid virus spread within orchards by movement of contaminated soil, irrigation water, seed coats from infected fruits and contaminated agricultural tools.
Significance
Among diseases affecting papaya, those caused by viruses cause the greatest losses worldwide. The most important viruses that infect papaya in Northeastern Brazil are Papaya ringspot virus (PRSV), family Potyviridae, genus Potyvirus; Papaya lethal yellowing virus (PLYV), genus Sobemovirus and the Papaya meleira virus (PMeV). As it is still being characterized, PMeV has not been classified by the International Committee on Taxonomy of Viruses (ICTV).
Papaya lethal yellowing disease is caused by PLYV and has been detected only in Northeastern Brazil. The virus was first detected in the state of Pernambuco, followed by Rio Grande do Norte, Paraiba and then Ceará. In the state of Ceará, the virus was first detected in the counties close to the Rio Grande do Norte and has not been detected yet in the state of Piaui (west of Ceará) nor in the Ceará counties close to the state of Piaui. The disease has become a serious problem for the papaya producers in the region because of its damage to crop production and its increasing spread throughout papaya. The origin of PLYV is unknown but the virus may have come from a wild native host under natural conditions or it could have resulted from a mutation of another plant virus that occurs in the region. The spread of the virus in the region is occurring from east to west.
Extensive surveys of the production areas of Ceará and Rio Grande do Norte revealed low incidence of PLYV in the papaya orchards visited. The virus was distributed in small patches of plants inside the orchards or on the border of the orchards. This kind of disease distribution indicates that the virus is likely introduced by infected plantlets or by contaminated tools and that it is subsequently spread to neighboring plants by grower activities.
Selected References
Camarço, R.F.E.A., Lima, J.A.A., and Pio-Ribeiro, G. 1998. Transmissão e presença em solo do "Papaya lethal yellowing virus". Fitopatologia Brasileira 23:453-458.
Daltro, C.B., Pereira, A.J., Cascardo, R.S., Alfenas-Zerbini, P., Beserra Jr, J.E.A., Lima, J.A.A., Zerbini, F.M., Zerbini, F.M., and Andrade, E.C. 2012. Genetic variability of papaya lethal yellowing virus isolates from Ceará and Rio Grande do Norte states, Brazil. Tropical Plant Pathology 37:37-43.
Gonsalves, D. 1998. Control of papaya ringspot virus in papaya: A case study. Annual Review Phytopathology 36:415-437.
Kitajima, E.W., Oliveira, F.C., Pinheiro, C.R.S, Soares, L.M., Pinheiro, K., Madeira, M.C., and Chagas, M. 1992. Amarelo letal do mamoeiro solo no estado do Rio Grande do Norte. Fitopatologia Brasileira 17:282-285.
Lima, J.A.A., Lima, A.R.T., and Marques, M.A.L. 1994. Purificação e caracterização sorológica de um isolado do vírus do amarelo letal do mamoeiro 'solo' obtido no Ceará. Fitopatologia Brasileira 19:437-441.
Loreto, T.J.G., Vital, A.F., and Rezende, J.A.M. 1983. Ocorrência de um amarelo letal do mamoeiro solo no estado de Pernambuco. O Biológico 49:275-279.
Nascimento, A.K.Q., Lima, J.A.A., Nascimento, A.L.L., Beserra, E.A.Jr., and Purcifull, D. 2010. Biological, physical, and molecular properties of a Papaya lethal yellowing virus isolate. Plant Disease 94:1206-1212.
Ramos, N.F., Nascimento, A.K.Q., Goncalves, M.F.B., and Lima, J. A. A. 2008. Presença dos vírus da mancha anelar e do amarelo letal em frutos de mamoeiro comercializados. Tropical Plant Pathology 33: 449-452.
Saraiva, A.C.M., Paiva, W.O., Rabelo Filho, F.A.C., and Lima, J.A.A. 2006. Transmissão por mãos contaminada e ausência de transmissão embrionária do vírus do amarelo letal do mamoeiro. Fitopatologia Brasileira 31: 79-83.
Silva, A.M.R., Kitajima, E.W., Souza, M.V., and Resende, R. 1997. Papaya lethal yellowing virus: a possible member of the Tombusvirus genus. Fitopatologia Brasileira 22:529-534
Teixeira, M.G.C.; Lima, J.A.A., Sousa, A.E.B.A., and Fernandes, E.R. 1999. Baixos graus de incidência do vírus do amarelo letal do mamoeiro em municípios do Rio Grande do Norte. Caatinga 12:29-33.
