Harrison, N.A. and M.L. Elliott. 2008. Lethal Yellowing of Palms.
The Plant Health Instructor. DOI: 10.1094/PHI-I-2008-0714-01
Lethal yellowing of palm
'Candidatus Phytoplasma palmae'
Palms (species in the family Arecaceae);
Pandanus utilis (Pandanaceae)
Authors
Nigel A. Harrison and Monica L. Elliott
University of Florida

Random pattern of lethal yellowing disease in Cocos nucifera plantation. |
During the last four decades, outbreaks of lethal yellowing disease have killed most of the once prevalent tall-type coconut cultivars in Florida (United States) and Jamaica. While coconuts are the most important economic palm affected by this disease, 35 other palm species are susceptible to lethal yellowing.
Symptoms and Signs
No single symptom is diagnostic of lethal yellowing. Symptoms are variable among palm genera and, in the case of coconuts, among cultivars. It is the pattern of appearance and chronological progression of symptoms that accurately identifies the disease. Confirmation of lethal yellowing is based on a molecular diagnostic assay using the polymerase chain reaction (PCR). At least 36 palm species (Table 1) have been documented as susceptible to lethal yellowing, but coconut palm (Cocos nucifera) is most vulnerable to the disease, followed by
Pritchardia species, Christmas palm (Adonidia merrillii), and date palm (Phoenix dactylifera).
Table 1. Palm species susceptible to lethal yellowing disease. |
|
Adonidia merrillii |
Dictyosperma album |
Phoenix dactylifera |
|
Aiphanes lindeniana |
Dypsis cabadae |
Phoenix reclinata |
|
Allagoptera arenaria |
Dypsis decaryi |
Phoenix rupicola |
|
Arenga engleri |
Gaussia attenuata |
Phoenix sylvestris |
|
Borassus flabellifer |
Howea belmoreana |
Pritchardia affinis |
|
Caryota mitis |
Howea forsteriana |
Pritchardia pacifica |
|
Caryota rumphiana |
Hyophorbe verschaffeltii |
Pritchardia remota |
|
Chelyocarpus chuco |
Latania lontaroides |
Pritchardia thurstonii |
|
Cocos nucifera |
Livistona chinensis |
Ravenea hildebrantii |
|
Corypha utan |
Livistona rotundifolia |
Syagrus schizophylla |
|
Crysophila warsecewiczii |
Nannorrhops ritchiana |
Trachycarpus fortunei |
|
Cyphophoenix nucele |
Phoenix canariensis |
Veitchia arecina |
The first obvious symptom on mature palms (those able to produce fruit) is a premature drop of most or all fruits. For coconuts, the calyx end of the nut (fruit) will usually develop a brown to black, water-soaked appearance (Figure 1). Nut or fruit-fall is accompanied or followed by flower necrosis. This symptom is most readily observed on newly mature flowers as they emerge from the spathe (Figure 2). Male flowers abscise, and no fruit is set.

Figure 1 |

Figure 2 |
The next symptom observed on mature palms (the first symptom for immature palms or non-fruit bearing palms) is foliar discoloration. This symptom varies markedly among coconut cultivars and other palm genera.
For tall-type coconut cultivars (e.g., ‘Jamaica Tall’), the foliage turns yellow, beginning with the lowermost (oldest) leaves and progressing until the entire crown is affected (Figure 3). In some cases, this symptom is first seen as a solitary, yellowed leaf (“flag leaf”) in the middle of the leaf canopy (Figure 4). Typically, yellowed leaves remain turgid, but eventually turn brown, desiccate, and hang down to form a skirt around the trunk for several weeks before falling. As leaf yellowing advances, the spear (youngest) leaf collapses and hangs down in the crown. Death of the apical meristem (bud) usually occurs when one-half to two-thirds of the crown has yellowed. Eventually, the entire crown of the palm withers and topples, leaving a bare trunk standing (Figure 5). Infected palms usually die within 3 to 5 months after the first appearance of symptoms.

Figure 3 |

Figure 4 |

Figure 5 |
For dwarf-type coconut cultivars (e.g., ‘Malayan Green Dwarf’), leaves generally turn a reddish to grayish-brown rather than yellow (Figure 6). Leaflets on the green form of the Malayan Dwarf cultivar may be folded around the midvein (Figure 7). Sometimes, affected leaves appear flaccid, giving an overall wilted appearance to the palm canopy.

Figure 6 |

Figure 7 |
Foliar yellowing also develops on
Caryota mitis (clustering fishtail palm),
C. rumphiana (Figure 8),
Chelyocarpus chuco (Figure 9),
Corypha utan,
Dictyosperma album (hurricane or princess palm) (Figure 10),
Livistona chinensis (Chinese fan palm),
Pritchardia spp., and
Trachycarpus fortunei (windmill palm). In contrast, successively younger leaves turn varying shades of reddish-brown to dark brown or gray in other palm species, such as
Adonidia merrillii (Christmas palm),
Borassus flabellifer (palmyra palm) (Figure 11),
Dypsis cabadae (cabada palm),
Phoenix spp. (date palm, Canary Island date palm, wild date palm) (Figure 12), and
Veitchia arecina (Montgomery palm). Differences may occur in the stage at which spear leaf collapse and necrosis appears on these species. For date palms and palmyra palm, death of the spear leaf often precedes foliar discoloration. For
Adonidia and
Veitchia spp., the spear leaf is usually not affected until after all other leaves have died.

Figure 8 |

Figure 9 |

Figure 10 |

Figure 11 |

Figure 12 |
Pathogen Biology
Lethal yellowing is caused by a phytoplasma, a cell wall-less bacterium that belongs to the class Mollicutes. The phytoplasma has been classified as a member of group 16S rDNA RFLP group 16SrIV, subgroup A (16SrIV-A). The proposed name for the pathogen is ‘Candidatus Phytoplasma palmae.’
The phytoplasma, which is not culturable, is found only in the phloem of host plants. When observed in phloem sieve elements by electron microscopy, the shape of phytoplasma cells varies from bead-like to filamentous (Figure 13). Non-filamentous forms average 295 nm in diameter and filamentous forms average 142 nm in diameter and at least 16 µm in length. Each phytoplasma cell is enclosed by a trilaminar unit membrane and contains cytoplasm with DNA strands and ribosomes.
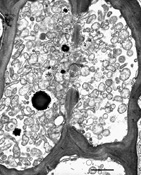
Figure 13 |
Molecular studies have determined that the lethal yellowing phytoplasma exists as a group of nearly identical strains in the western Caribbean region. Collectively, these strains are phylogenetically distinct from phytoplasmas that infect coconut in Africa or southeast Asia. The lethal yellowing phytoplasma is most closely related to, but distinct from, phytoplasmas associated with decline-type diseases of the monocot
Carludovica palmata (Cyclanthaceae) in Yucatán, Mexico,
Phoenix canariensis (Canary Island date palm) in the Corpus Christi area of southern Texas (United States), and phytoplasmas causing a newly recognized coconut leaf yellowing syndrome in southwestern Mexico.
Disease Cycle and Epidemiology
Experimental evidence implicates the planthopper
Myndus crudus as a vector of the lethal yellowing phytoplasma (Figure 14). The planthopper is an insect with piercing and sucking mouthparts, and feeds on the contents of the plant host vascular system. The insect spreads the phytoplasma during feeding activity as it moves from palm to palm. The phytoplasma is not known to survive outside either its plant or insect hosts. The geographic range of lethal yellowing is limited in the United States to the subtropical southern third of Florida because the planthopper is not considered cold hardy.

Figure 14 |
Inoculation of a susceptible plant initiates infection that is followed by a prolonged latent (incubation) phase estimated between 112 to 262 days. About 80 days prior to symptom appearance, the growth of infected palms is stimulated. This is followed by a period of gradual decline, and growth ceases about 1 month before the end of the incubation phase.
Following an initial disease outbreak, further spread of lethal yellowing is characterized by a “jump-spread” pattern, indicating dissemination involving an airborne vector. Spread occurs among susceptible palms within a localized area, resulting in a random pattern around an active focus of disease (Figure 15) that eventually claims most susceptible palms within the locality (Figure 16). Beyond this primary focus, further spread may occur in jumps of a few to 100 km or more, thus establishing new disease foci. Differences in the rates of spread of lethal yellowing at different geographical locations have also been noted. In Florida (United States), spread of the disease from the cities of Miami to Palm Beach, a distance of about 128 km, occurred within 3 years. In Jamaica, however, movement of the disease from the west to the east end of the island, a distance of approximately 238 km, took about 60 years.

Figure 15 |

Figure 16 |
Disease Management
To discourage the spread of lethal yellowing in the tropics, commercial movement of living palms from locations affected by lethal yellowing to disease-free areas is generally not permitted. However, quarantine requirements vary according to the specific geographical areas involved. Technical guidelines for the safe movement of coconut germplasm have been developed under the auspices of the FAO International Board for Plant Genetic Resources.
Chemical control of lethal yellowing is accomplished with the antibiotic oxytetracycline HCl (Terramycin), which is administered to palms as a liquid injection into the trunk. As a therapeutic measure, systemic treatment on a 4-month treatment schedule should begin as early as possible after the onset of symptoms. Palms with >25% discolored leaves should be removed, since they are unlikely to respond to Terramycin treatment. The antibiotic can also be used preventively to protect palms when lethal yellowing is known to occur in the area. The dosage recommended depends on the size of the treated palm. The approximate cost of Terramycin ranges from $1.50 to $4.00 per palm per treatment, depending on the number of palms treated. Control of planthopper populations with insecticides is currently insufficient to justify repeated applications in landscapes or palm plantations.
Use of host resistance represents the most practical long-term tool for managing lethal yellowing. Many palm species are not susceptible to lethal yellowing and provide important alternative choices for ornamental landscape plantings. To date, lethal yellowing has not been reported on most palm species native to Florida and the Caribbean Basin. These include
Sabal palmetto (cabbage palm),
Roystonea regia (royal palm),
Acoelorrhaphe wrightii (Paurotis or Everglades palm), and
Thrinax species (thatch palms). On the other hand,
Cocos nucifera (coconut),
Pritchardia spp.,
Adonidia merrillii (Christmas palm), and
Phoenix dactylifera (date palm) have sustained consistent losses and are not recommended for widespread landscape use in areas where lethal yellowing occurs.
Coconut cultivars, such as the ‘Malayan Dwarf’ or hybrid ‘MayPan’ (‘Malayan Dwarf’ x ‘Panama Tall’), have exhibited acceptable levels of resistance in most areas. However, recent reports of lethal yellowing losses in ‘Malayan Dwarf’ and ‘MayPan’ of 70% and 83%, respectively, at localized sites in southeastern Florida and 95 to 99% in Jamaica cast doubt on the long-term resistance of these cultivars.
Significance
While coconut palm is highly valued as a woody ornamental plant in the United States, it is an important subsistence crop in the coastal tropics. Almost all parts of the coconut palm are used, providing food, drink, fuel, shelter, and cash income for producers. The term lethal yellowing (often shortened to LY) was first used in the mid-1950s to describe a fatal disease of unknown etiology that had affected coconuts in western Jamaica since the 1800s.
During the last four decades, outbreaks of lethal yellowing disease have killed most of the once prevalent tall-type coconut cultivars in both Jamaica and Florida (United States). The disease has also been reported from the Bahamas, Belize, the Cayman Islands, Cuba, Dominican Republic, Guatemala, Haiti, Honduras, Leeward Islands (Nevis), and southern Mexico (Yucatan peninsula).
Recurrent coconut diseases that resemble lethal yellowing have been recorded elsewhere in the tropics under a variety of names depending on location. Collectively referred to as “lethal yellowing-type diseases,” they include Awka wilt (Nigeria), Cape St. Paul wilt (Ghana), Kaïncopé (Togo), and Kribi (Cameroon) in West Africa; lethal disease (Kenya, Tanzania and Mozambique) in East Africa; and Kalimantan wilt (Central Kalimantan), Natuna wilt (Natuna Islands), and Malaysian wilt (Peninsula Malaysia) in South East Asia.
Selected References
Broschat, T.K., N.A. Harrison, and H. Donselman. 2002. Losses to lethal yellowing cast doubt on coconut cultivar resistance. Palms 46:185-89.
Eden-Green S.J. 1997. History, distribution and present status of lethal yellowing-like disease of palms. Pages 9-25 in: Proceedings of an International Workshop on Lethal Yellowing-Like Diseases of Coconut, Elmina Ghana, November 1995. S. J. Eden-Green and F. Ofori, eds. Natural Resources Institute, U.K.
Frison, E.A., C.A.J. Putter, and M. Diekmann. 1993. Technical Guidelines for the Safe Movement of Coconut Germplasm. Food and Agriculture Organization/International Plant Genetic Resources Institute, Rome.
Harrison, N.A., I. Cordova, P. Richardson, and R. DiBonito. 1999. Detection and diagnosis of lethal yellowing. Pages 183-196 in: Current Advances in Coconut Biotechnology. C. Oropeza, J.L. Verdeil, G.R. Ashburner, R. Cardeña, and J.M. Santamaría, eds. Kluwer Academic Publishers, Dordrecht, The Netherlands.
Harrison, N.A., W. Myrie, P. Jones, M.L. Carpio, M. Castillo, M.M. Doyle, and C. Oropeza. 2002. 16SrRNA interoperon sequence heterogeneity distinguishes strain populations of palm lethal yellowing phytoplasma in the Caribbean region. Annals of Applied Biology 141:183-193.
Harrison, N.A., M. Narváez, H. Almeyda, I. Cordova, M.L. Carpio, and C. Oropeza. 2002. First report of group 16SrIV phytoplasmas infecting coconut palms with leaf yellowing symptoms on the Pacific coast of Mexico. Plant Pathology 51:808.
Howard, F.W., R.C. Norris, and D.L. Thomas. 1983. Evidence of transmission of palm lethal yellowing agent by a planthopper,
Myndus crudus (Homoptera, Cixiidae). Tropical Agriculture, Trinidad 60:168-171.
IRPCM Phytoplasma/Spiroplasma Working Team – Phytoplasma Taxonomy Group. 2004. ‘Candidatus Phytoplasma’, a taxon for the wall-less non-helical prokaryotes that colonize plant phloem and insects. International Journal of Systematic and Evolutionary Microbiology 54:1243-1255.
Lee, I.-M., R.E Davis, and D.E. Gundersen-Rindal. 2000. Phytoplasma: phytopathogenic mollicutes. Annual Review of Microbiology 54: 221-255.
McCoy, R.E. 1982. Use of tetracycline antibiotics to control yellows diseases. Plant Disease 66:539-542.
McCoy, R.E., F.W. Howard, J.H. Tsai, H.M. Donselman, D.L. Thomas, H.G. Basham, R.A. Atilano, F.M. Eskafi, L. Britt, L., and M.E. Collins. 1983. Lethal Yellowing of Palms. Agricultural Experiment Station Bulletin No. 834, Institute of Food and Agricultural Sciences, University of Florida, Gainesville, FL.
Mejía, F., M. Palmieri, C. Oropeza, M. Doyle, N. Harrison, E. Aguilar, M. Narváez, R. Estrada, and G. Ortiz, G. 2004. First report of coconut lethal yellowing disease in Guatemala. Plant Pathology 53:800.
Myrie, W.A., L. Paulraj, M. Dollet, D. Wray, B.O. Been, and W. McLaughlin. 2006. First report of lethal yellowing disease of coconut palms caused by phytoplasma on Nevis Island. Plant Disease 90:834.
