Dwarf mistletoes
Arceuthobium species
Many gymnosperms (conifers)

Southwestern dwarf mistletoe (Arceuthobium vaginatum subsp.
cryptopodum) with bicolored, mature fruits on fine, bent pedicels that attach fruits to shoots. [courtesy D. Conklin] |
Symptoms and Signs
Dwarf mistletoe shoots are macroscopic and symptoms of advanced infections are often obvious. Especially in early stages of infection, however, symptoms and signs may be subtle or absent.
Symptoms
The first symptom of infection is
swelling of a branch or stem near the point of infection (Figure 2). The swelling is usually fusiform (tapered at the ends) and the degree of swelling varies. Some dwarf mistletoes cause cankers in older stem infections, which may serve as infection courts for stem-decay fungi (Figure 3).
The most obvious symptom of dwarf mistletoe infection is a
witches’ broom (Figure 4) forming after a branch has been infected for several years or more. This is a dense mass of profuse, sometimes distorted, branches that form in response to infection by dwarf mistletoe. A few species of dwarf mistletoes do not induce witches’ broom formation.

Figure 2 |

Figure 3 |

Figure 4 |
Crown symptoms include the visual effects of dwarf mistletoe on tree vigor. Upper crowns may thin and eventually die in trees that are severely infected, particularly with one or more large witches’ brooms in the lower crown (Figure 5). The brooms are nutrient sinks, disrupting the physiology of the tree and robbing the top of nutrients. Eventually the entire crown may thin, leading to
mortality (Figure 6). Witches’ brooms are often the last part of the tree to die.

Figure 5 |

Figure 6 |
Signs
Shoots are the best indication that a tree is infected by a dwarf mistletoe (Figure 7). Shoots usually develop 3-5 years after a branch is infected. Depending on the species of dwarf mistletoe, shoots may be brown, orange, green, yellow, olive, or yellow-green. The shortest dwarf mistletoe shoots in the Americas are those of eastern dwarf mistletoe,
A. pusillum; with shoots about 1 cm tall (Figure 8); the most massive plants in the genus are those of
A. globosum subsp.
grandicaule in Guatemala, whose shoots can exceed 70 cm (28 inches) in length (Figure 9). At each node is a pair of small, scale-like leaves (Figure 10). Shoots typically live for 5 to 7 years before they die and abscise. The plant stays alive and typically produces new shoots, but there may be periods when shoots are hard to find, such as during a drought. In such cases, residual
basal cups may be present where shoots have abscised (Figure 11).

Figure 7 |

Figure 8 |

Figure 9 |

Figure 10 |

Figure 11 |
Pathogen Biology
The genus
Arceuthobium (Santalales: Viscaceae) is a group of small plants that are exclusively parasitic on conifers. The name is from Greek (arkeuthos +
bios) and means juniper-life; the first species described was on juniper in Europe. There are about 42 species, mostly in North and Central America, where they occur only on members of the family Pinaceae. Eight species occur in Eurasia and Africa, where some are also parasitic on junipers (family Cupressaceae).
Arceuthobium species tend to be host-specific, parasitizing only one or two principal hosts, but there are some exceptions; for example,
A. globosum subsp.
grandicaule parasitizes more than ten species of pine in Mexico. Some of the more important mistletoes in North America are
-
A. abietinum, on true firs
-
A. americanum, on lodgepole pine
-
A. campylopodum, on ponderosa and Jeffrey pines
-
A. douglasii, on Douglas-fir
-
A. pusillum, on eastern spruces
-
A. tsugense, on hemlocks
-
A. vaginatum, on southwestern and Mexican pines
Endophytic system
The endophytic system, as the name implies, is the portion of the dwarf mistletoe that is inside the host plant. It consists of two components that provide anchorage and extract nutrients from the host—cortical strands and sinkers (Figure 12).
Cortical strands are fine strands of mistletoe tissue that ramify through the host phloem and give rise to shoots and sinkers. Older portions of the system may be pushed with the old phloem into the periderm (outer bark). In systemic infections, strands continue acropetal growth (towards the shoot apex) to the apical meristem of the host branch (shoot tip). There growth keeps up with branch elongation and new branches are also infected.
Sinkers are strands of mistletoe tissue that are embedded in the rays of the xylem (wood). They are initiated near the growing tips of the cortical strands and grow radially, towards the host vascular cambium. Here they displace cambium initials. When the cambium produces new xylem and phloem, the sinkers maintain pace via an intercalary meristem aligned with the cambium. In this way, without actively penetrating the xylem, the sinkers become embedded in the rays and avoid being torn apart by the host’s growth (Figure 13).

Figure 12 |
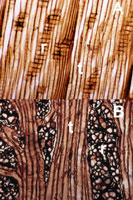
Figure 13 |
Local vs. systemic infection
There are two types of infections by dwarf mistletoes:
Local infection: The endophytic system does not advance far from the point of infection. Mistletoe shoots are produced only near the point of infection, and swelling is usually persistent (Figure 2). All species can produce this kind of infection.
Systemic infection: The endophytic system grows to the apical meristem of the infected shoot. It keeps pace with apical shoot growth and invades all branches subsequently produced from it (Figure7). Swelling may occur in early stages but it often becomes less pronounced or disappears over time. Mistletoes with systemic infection often produce very large brooms. Systemic infection is thought to be an apomorphic trait (i.e., species that cause systemic infection evolved from ancestors that caused local infection) and occurs regularly in only a few
Arceuthobium species:
A. americanum, A. douglasii, A. guatamalense, A. minutissimum, and
A. pusillum. Systemic infection tends to be associated with other apomorphic traits such as high host specificity, small shoot size, flabellate (fan-shaped) vs. verticillate (whorled) branching, and formation of flower buds in late summer for early spring flowering.

Figure 2 |

Figure 7 |
Host susceptibility
For each dwarf mistletoe species, hosts are ranked in classes of susceptibility (Table). The classes are defined on the basis of percentage infection when within 6 m of a heavily infected host.
Table. Host susceptibility classes for dwarf mistletoes.
|
Class |
Incidence of infection * |
| Principal | >= 90 % |
| Secondary | 50-90 % |
| Occasional | 5-50 % |
| Rare | <= 5 % |
| Immune | 0 % |
* Percentage of trees infected when within 6 m of heavily infected principal hosts in stands older than 30 years.
Parasitism
Dwarf mistletoes are obligate parasites, meaning they cannot survive (except as seed) without a living host. From the host they receive all of their water and inorganic nutrients, and most of their fixed carbon.
Dwarf mistletoe shoots (especially fruits) transpire much more water, on a surface-area basis, than does host foliage. The difference may increase up to 60-fold during periods of water stress, when the host limits its own water use. Most inorganic nutrients are in higher concentration in dwarf mistletoe shoots than in host tissues.
Although dwarf mistletoes can photosynthesize to a limited extent, they derive almost all their fixed carbon from their hosts. In contrast, the leafy, or true, mistletoes depend on their host mostly for water and minerals and less for photosynthates.
Dwarf mistletoes apparently establish themselves as a sink for fixed carbon by the production of hormones. Most attention to date has focused on cytokinins. This not only satisfies the need of the parasite, it also leads to lush development and often profuse branching of nearby host tissue. The resulting witches’ brooms are produced at the expense of other parts of the tree, especially the upper crown and perhaps the roots. This disturbance of host physiology profoundly affects growth and can eventually lead to mortality. Yet the witches’ brooms are often the last part of a tree to die.
Reproduction and dispersal
Although photosynthesis by shoots may contribute slightly to dwarf mistletoe nutrition, the primary function of shoots is reproduction. Dwarf mistletoes are dioecious; they produce male and female plants.
The mature fruit contains a single seed that is explosively discharged by one of the most effective hydrostatic mechanisms among flowering plants. During maturation, the fruit pedicel (Figure 1) bends so that the seed is often discharged at about 30 degrees above the horizontal, an angle that maximizes lateral distance for targets within 11 vertical meters (36 feet) below the source, and also allows the possibility of climbing the tree canopy. The initial velocity of the seed is about 27 m•sec-1 (60 mph) (Figure 14). Maximum dispersal distance is about 16 m (52 ft), but dispersal distances of 10 m (33 ft) or less are more typical. The seed is coated with a sticky mucilage (viscin), so it adheres to needles that it strikes (Figure 15). It remains on the needle until rain wets the viscin, allowing the lubricated seed to slide down the needle and, if the needle is upright, make contact with the bark and needle base.

Figure 1 |

Figure 14 |
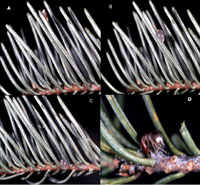
Figure 15 |
Disease Cycle and Epidemiology
Disease Cycle
The dwarf mistletoe life cycle commonly takes 6 to 8 years (Figure 16). As the seed germinates on host shoots, the young radicle contacts the host bark, often beside a needle fascicle, and forms a disk-like holdfast that enlarges and grips the bark tightly. From it, a wedge develops and penetrates the bark. Penetration continues to the cambium and stops. From this penetration peg, cortical strands begin to grow in the bark, initiating development of the endophytic system (see Pathogen Biology section).
![Figure 16. Life cycle of lodgepole dwarf mistletoe. [provided by W. Jacobi]](/edcenter/disandpath/parasiticplants/pdlessons/PublishingImages/Dwarfmistletoes16sm.jpg)
Figure 16 |
Infection generally occurs in host shoots that are one to five years old. That is because such shoots are most likely to have needles that intercept seeds, but also because the bark is thin enough to be penetrated before the germinating seed depletes its resources.
About 3 to 5 years after infection, after the endophytic system is developed, the mistletoe plant begins to produce aerial shoots (see Symptoms and Signs section). Shoots typically live for 5 to 7 years and may produce several crops of flowers before they die and abscise. The plant stays alive inside the host, however, and typically produces new shoots repeatedly for many years. Flowering occurs either in early summer or late summer/fall. The mature seed is explosively dispersed (see Pathogen Biology).
Many seeds are intercepted within the originating crown but some are intercepted by the crown of adjacent susceptible trees. Most seeds fail to reach a site suitable for infection; a rare few seeds are successfully transported by animal vectors to establish new infestations.
Effects of stand conditions and history
Fire may be the most important factor affecting the distribution and abundance of dwarf mistletoes in most forest types of the western United States. The fire regime in general and most recent fire in particular determines whether fire reduces or increases mistletoe. A burn that kills infected trees reduces the population of dwarf mistletoe, at least in the short term. In most cases, trees re-colonize the site much more quickly than does the dwarf mistletoe. Large, continuous, stand-replacing fires substantially reduce dwarf mistletoe populations across the landscape over long periods (Figure 17) and may eliminate local populations and result in new stands that are disease-free to maturity. Patchy burns also reduce dwarf mistletoe populations, but scattered, infected trees that escape the fire provide inoculum for early infection of new regeneration. Because infected trees may survive even extensive burns, fire can also increase mistletoe in the long term.
Historic practices can have strong influences on dwarf mistletoes. As might be inferred from the discussion of fire above, fire suppression and exclusion can lead to increase in the distribution and severity of dwarf mistletoe (Figure 18). Harvest practices that emulate fire, such as clearcuts, can have the opposite effect. Other silvicultural approaches, when carefully designed with mistletoe in mind, can have long-lasting effects in reducing severity (see Disease Management section). On the other hand, partial harvesting that does not address mistletoe, even when it is silviculturally appropriate in healthy stands, can have profound effects by creating the multistory structure that favors intensification and spread of mistletoe. Several large-scale surveys, repeated during the 20th century, suggest that there was a gradual increase in incidence of dwarf mistletoe in the areas studied (Figure 19).
Stand structure is important in determining the rate of spread and intensification of dwarf mistletoe. When small trees are growing under large, infected trees, dispersal distance can be greater than in even-aged stands. The small trees can be infected in their upper crowns at a young age, which leads to severe disease and early mortality.
Composition certainly can affect the spread and intensification of mistletoes. In stands with both host and nonhost species, host trees are usually more isolated from other host trees and nonhost trees can act as barriers to seed dispersal—both conditions tend to reduce mistletoe spread.
Host vigor has mixed effects. Dwarf mistletoe plants on vigorous hosts, especially in open stands, grow larger and produce more seed than those on less vigorous hosts. Dense crowns of vigorous hosts tend to intercept more seeds and thus infection may be more likely. On the other hand, the host grows in height faster, and vertical spread of the parasite is impeded by dense foliage. Thus, vigorous trees can sometimes keep infection in the lower crown and escape serious damage.

Figure 17 |

Figure 18 |

Figure 19 |
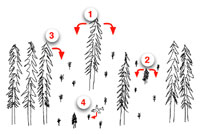
Figure 20 |
Sources of inoculum
When reduction of dwarf mistletoe is a goal in a managed forest, it is useful to consider sources of inoculum in four categories (Figure 20). These sources should be considered before any management actions in infested stands:
- The most potentially damaging inoculum typically comes from residual infected trees over developing regeneration (see Stand structure, above). This situation could arise naturally from partial killing of an overstory, but also from partial cutting that leaves infected, mature trees in place.
- Infected, advanced regeneration can cause similar damage, although dispersal distance from such trees is less. These are often noncommercial trees that are left after treatment of the overstory. Even if the overstory is completely sanitized, the understory must be treated also if it contains infected trees.
- Inoculum from residual, infected stands at the edges of disease-free stands can also be significant. Carefully selecting boundaries or size of treatment units can reduce the effect of such inoculum (see Disease management section).
- A variety of animals, particularly birds, have been implicated in the dispersal of the sticky seeds of dwarf mistletoes. This probably accounts for the occurrence of the disease in isolated stands and disease centers in large, regenerated, disease-free stands. However, most workers feel that such inoculum is rare enough to be epidemiologically insignificant within the time frame of forest management.
Disease Management
Development of management approaches for dwarf mistletoes can be considered one of the first success stories of forest pathology in North America. Early western pathologists provided principles and approaches for control of dwarf mistletoes through forest management. However, it gradually became clear that, although control of dwarf mistletoes was simple in theory, it was sometimes complex and difficult in practice.
Features of dwarf mistletoes that facilitate their management include the following:
-
Dwarf mistletoes are obligate parasites. They cannot survive without a living host. Once the branch or tree dies or is cut, the parasite dies.
-
Dispersal distance is limited and spread is slow. Explosive seed dispersal is only up to about 60 ft from a tall, isolated tree. In single-storied stands, spread is usually about 2 ft per year. This creates possibilities for protecting trees by separation from infected trees.
-
The life cycle is long. From dispersal to production of a new generation of mature fruit typically takes 6–8 years. Disease intensification (multiplication of infections and increase in severity) is therefore fairly slow in infested areas.
-
Dwarf mistletoes tend to be host specific. Mixed stands and changes in composition therefore can create a disadvantage for the mistletoes.
-
They are relatively easy to detect. Unlike most pathogens, dwarf mistletoes are entirely above ground, partly exposed on the surface of the host, visible without a microscope, and usually cause distinctive symptoms.
Dwarf mistletoe rating
Hawksworth’s 6-class dwarf mistletoe rating (DMR) system is used almost universally to rate severity of dwarf mistletoe (Figure 21). Many disease parameters and management recommendations are provided in terms of DMR. Actually a 7-class system since it ranges from 0 (uninfected) to 6, it is based on rating each third of the crown on a scale from 0–2, then summing for the tree rating. In recent applications, stem infections are only considered if there are no branch infections at all, in which case the tree is scored “1”. Stem infections usually contribute little to effective spread.
In a check of rating accuracy by cutting and examining tree crowns, ratings were accurate about 75% of the time. Ratings were more reliable for small than for large trees. Severely infected trees tended to be underrated because of failure to detect infected branches in the upper crown. Lightly infected trees tended to be overrated because of a tendency to lower the boundary between the lower and middle third. These rating errors tended to cancel each other out, although overall there was a slight underestimate. Binoculars should be used to enhance detection. A common mistake is to stand too close to the tree, which can obscure symptoms and signs as well as cause perspective errors in dividing the crown into thirds.
Stand DMR as a single measure of disease intensity is calculated by averaging the DMR of all principal host trees in the stand, including uninfected trees. The percent of host trees that are visibly infected provides a measure of mistletoe incidence; the average DMR of infected trees only is designated dwarf mistletoe index (DMI) and complements percent infected with a relative measure of mistletoe severity on those trees.
Management approaches
Chemical and biological controls appear to be inadequate in most cases. Herbicides that kill the parasite, including the endophytic system, usually damage the host as well. Ethephon formulations (active ingredient 2-chloroethyl phosphoric acid, which releases ethylene) have been used successfully to cause abscission of shoots (Figure 22). They do not affect the endophytic system, however, so application must be repeated every few years to prevent inoculum production. A variety of interesting insects and pathogens attack dwarf mistletoes, but none are yet developed sufficiently for practical application (Figure 23).

Figure 21 |

Figure 22 |

Figure 23 |
In forest settings, then, silviculture is almost the sole toolkit for dwarf mistletoe management. Detailed considerations cannot be presented here, but following are some approaches that are used:
-
Choose uninfested borders for treatments. Regardless of stage of stand development or the management approach, treated areas should be bordered as much as possible by areas that will not be sources of inoculum (Figure 24). This includes nonsusceptible or uninfested stands, roads, forest openings, etc.
-
Size matters. To avoid re-infestation of treated areas that have infested stands on the border, treated areas must be large enough that spread into them from the borders is insignificant, or at least acceptable, during the life of the stand (Figure 25). The proportion of a treated area affected by mistletoe from the border decreases as the treated area becomes larger. In this situation, 20 acres is considered a minimum and 40 acres is recommended. As patches increase beyond 40 acres, the advantage of increasing size becomes less. Irregularly shaped or long, narrow patches must be larger to have a similar area protected.
-
Favor nonhosts. Whether planting, spacing, thinning, selecting trees to provide seed for the next generation, etc., encourage and favor tree species that are not hosts of the mistletoe in the stand (Figure 26).
-
Grace period for seedlings. Because of their small size as targets and their short exposure to inoculum, seedlings generally can be considered safe from infection until they are 10 years old or 3 feet tall, whichever comes first. Infection of smaller or younger trees does occur, but it is generally rare. This gives some time before infected overstories must be treated after regeneration is established.
-
Even-aged management. Even-aged management is regulation of age structure through removal and regeneration treatments such that trees are of similar age. Where silviculturally appropriate, even-aged management, especially at the stage of regeneration, offers the best opportunity to establish a mistletoe-free stand. This means cutting or killing the stand, either all at once (e.g., clearcut) or in stages by seed-tree method (cutting of all trees except for a few desirable individuals that provide seed for the next generation) or shelterwood method (cutting of all trees in a series of two or more over a relatively short period of time, establishing even-aged reproduction under the partial shelter of the previous generation). In seed-tree or shelterwood methods, residual overstory trees must be removed before the regeneration is out of its grace period (Figure 27).
-
Sanitation. Sanitation, the removal or killing of infected trees to protect other trees, is important in many kinds of stands at various developmental stages. “Sanitation cutting” (or simply sanitation) has been distinguished from “sanitation thinning.” Sanitation cutting is the attempted removal of all visibly infected trees, though it usually is combined with thinning goals also. In sanitation thinning, the emphasis is on spacing, and only the most severely diseased trees may be removed. Because of the long period between infection and appearance of symptoms (latency) and difficulty of locating every visibly infected tree, multiple entries (5 to 10 years apart) are usually necessary to sanitize a stand or minimize disease severity.
-
Prescribed fire. Species that regenerate well after fire, such as lodgepole pine, can be treated with prescribed, stand-replacing fire to establish a new stand in the absence of inoculum. This can be an effective and economical means of stand replacement, and it is entirely consistent with the natural disturbance regime. It can be used in heavily infested stands that have little economic value, or after merchantable trees are harvested. Stands should be inspected following the fire and any surviving trees felled. Even low-severity fire (such as a prescribed burn to reduce fuel loads) may be beneficial because infection centers tend to torch and lower branches, most likely to be infected, are often killed or pruned by scorch (Figure 28).
-
Pruning. Pruning may have several objectives. Pruning of large brooms, which are generally in the lower crown, can allow trees to recover vigor and substantially prolong their life (Figure 29). It is most often used in developed recreation sites. Another objective of pruning is sanitation (i.e., sanitation pruning). In this case the removals are intended to reduce the population and impact of dwarf mistletoes as well as future inoculum. Pruning can also be used to reduce hazard of personal injury or property damage from the fall of a large broom (especially on species with brittle wood, such as larch).

Figure 24 |
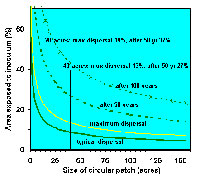
Figure 25 |
|

Figure 26 |

Figure 27 |
![Figure 28. Ponderosa pine with southwestern dwarf mistletoe killed by foliage scorch from surface fire. [courtesy B. Geils]](/edcenter/disandpath/parasiticplants/pdlessons/PublishingImages/Dwarfmistletoes28sm.jpg)
Figure 28 |

Figure 29 |
Significance
Effects on trees
In many parts of the western United States, dwarf mistletoes are the most important group of diseases. In terms of timber volume, annual losses have been estimated at 11.3 million cubic meters of wood in the western U.S. and 3.8 million cubic meters in western Canada. The U.S. losses equate to about 3.3 billion board feet, which has a value of roughly $1.4 billion and is enough wood to build up to 1,000,000 homes per year!
The impact of dwarf mistletoe is greatest when trees are infected at a relatively young age. Data from intensive studies of lodgepole pine dwarf mistletoe in Colorado have shown that stands infected at an early age have only 12.4% the volume of healthy stands by age 75, and the difference does not decrease thereafter (Figure 30).
However, impacts only become substantial when half or more of the tree’s crown is affected. This is equivalent to DMR (dwarf mistletoe rating, see dwarf mistletoe rating) of 3 or greater. One should also consider, however, that on a stand basis when average DMR reaches about 2, over 80 percent of trees are probably infected, leaving few choices for retaining trees with good potential growth and survival.
Although mortality is generally a less important effect than growth reduction, it can be substantial. It is difficult to quantify because of indirect effects. Infested stands have smaller trees with thinner crowns, so there is often greater establishment of seedlings in the understory. Also, mortality due to suppression is reduced. Nevertheless, mortality due to dwarf mistletoe (based on standing dead trees, after subtracting the values for comparable healthy stands) can be 15-20% of stems in heavily infested stands. Mortality is greater on dry than on wet sites, and is greater during or following drought. In some situations, bark beetles preferentially attack trees with mistletoe.
An illustration of the effects of dwarf mistletoe, based on output from the Forest Vegetation Simulator and Dwarf Mistletoe Impact Model, is presented here. Numeric projections are transferred to the Stand Visualization System which produces a spatial, graphical animation (Figure 31). In a healthy stand, basal area (cross-sectional area of each tree at 4.5 feet, summed over an acre) increases greatly. In an infested stand, DMR rises and basal area fails to increase and may even decrease over time due to mortality and reduced growth (Figure 32). Clearly, dwarf mistletoe greatly impacts not only commercial value, but also stand conditions that influence other vegetation and animals.
A third effect of dwarf mistletoes is reduction in the number and viability of seeds produced by the host. Seed production is not much affected by light infection, but moderate infection of ponderosa pine reduced seed production to 42% of healthy trees (by weight); severe infection reduced it to 29% of healthy trees. When viability is considered, the effect is somewhat greater.
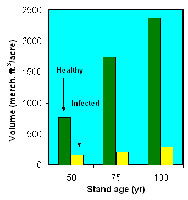
Figure 30 |

Figure 31 (Click on figure to see an animation of disease development) |
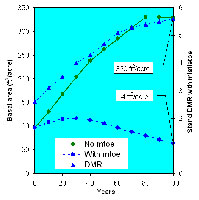
Figure 32 |
Forest dynamics
Most dwarf mistletoes occur primarily on early-seral species (i.e., occurring in early stages of a successional sequence). By killing and suppressing the growth of their hosts, the pathogens can sometimes promote succession to later-seral species that may be present. For example, lodgepole pine stands may be invaded by a spruce understory, and the turnover would be hastened by debilitation of the overstory.
However, dwarf mistletoes tend to increase the intensity of fire and the likelihood of a surface fire becoming a stand-destroying crown fire. Although in the short term this kills the obligate parasite, such fires usually result in re-establishment of the early-seral species, ensuring more host material for future generations of dwarf mistletoe.
Infected trees often have large witches’ brooms in the lower crown, persisting after the live crown has been raised in healthy trees. These brooms, full of resin and dense accumulations of live and dead needles, act as fuel ladders that increase the opportunity for a surface fire to torch or become a crown fire (Figure 33). Numerous observers have noted selective torching of infected trees during a surface fire. In general, more heavily infested stands have higher total fuel loading than uninfested stands.

Figure 33 |
Kissing under the mistletoe
A Christmas tradition is to hang mistletoe over a doorway (Figure 34). Amorous individuals may then kiss someone they catch under the mistletoe. However, this tradition is based on true, or leafy, mistletoes rather than dwarf mistletoes. The most common types of these have relatively large, white berries and prominent leaves.
The tradition is ultimately based on the European mistletoe,
Viscum album (Figure 35). As legend has it, the Druids, priests of the ancient Celtic culture of Europe, thought that mistletoe was sent to Earth by God because it occurred on the sacred oak trees. As a parasite, it represented human dependence on God. They recognized that the mistletoe thrush carried the mistletoe seeds and considered them to be messengers of God. Because it came as a gift from God, mistletoe had spiritual power. One way of using that power was to hang it over the doorway in winter to keep evil spirits from entering. This was especially helpful in winter because of the darkness and cold. No doubt the bright, evergreen foliage was a festive addition to winter décor as well.
The kissing part of the custom is probably related to the traditional association of mistletoe with fertility, but perhaps also to the fact that mistletoe was associated with the Norse goddess of love, Frigga. Part of the custom that is sometimes conveniently forgotten today limits its usage. Supposedly one should remove a berry from the plant for each kiss stolen from someone beneath it. When the berries are gone, so is the opportunity for kissing!
The similar American true mistletoes, most commonly used for this tradition here, are in the genus
Phoradendron (Figure 36).
Dendron in Greek means “tree” and
phora means "to bear", or in this case "thief", thus “tree thief.” They are larger than
Arceuthobium spp. and most have substantial leaves (though most that occur in deserts have greatly reduced leaves). They also differ in that the fruits are large, fleshy, and not explosive. They are dispersed by birds that feed on them. An APS Education Center article, Introduction to Parasitic Flowering Plants, has more information on the various types of mistletoes and other parasitic plants (http://www.apsnet.org/edcenter/intropp/PathogenGroups/Pages/ParasiticPlants.aspx).
More on this tradition, with lavish illustrations, can be found here:
What Does Mistletoe Have To Do With Christmas?
http://www.apsnet.org/publications/apsnetfeatures/Pages/Mistletoe.aspx
Traditions and legends - Introduction
http://www.mistletoe.org.uk/home/mtoetraditions.htm
Or search the annotated bibliography at the Mistletoe Center using the keyword folklore
http://www.rms.nau.edu/mistletoe/dyn/mtbib.shtml
![Figure 34. An antique, traditional greeting card. [photo from Forest Pathology, Flagstaff]](/edcenter/disandpath/parasiticplants/pdlessons/PublishingImages/Dwarfmistletoes34sm.jpg)
Figure 34 |

Figure 35 |

Figure 36 |
Selected References
Dixon, G.D., compiler. 2002. Essential FVS: A User’s Guide to the Forest Vegetation Simulator. Internal Report, USDA Forest Service, Forest Management Service Center, Fort Collins, CO. 204 pp. (Last revised January 2006).
 http://www.fs.fed.us/fmsc/ftp/fvs/docs/gtr/EssentialFVS.pdf
http://www.fs.fed.us/fmsc/ftp/fvs/docs/gtr/EssentialFVS.pdf
Geils B.W., J. C. Tovar, and B. Moody 2002. Mistletoes of North American conifers. General Technical Report RMRS-GTR-98. Ogden, UT: USDA Forest Service, Rocky Mountain Research Station. 123 p.
 http://www.fs.fed.us/rm/pubs/rmrs_gtr098.pdf
http://www.fs.fed.us/rm/pubs/rmrs_gtr098.pdf
David, L. (ed.). 2010. Dwarf mistletoe impact modeling system, User guide and reference manual, Nonspatial model, 2005 update. Unpublished report. Fort Collins, CO: USDA Forest Service, Forest Health Protection, Forest Health Technology Enterprise Team. 74 p.
 http://www.fs.fed.us/foresthealth/technology/pdfs/DM_User_Guide_MAG-95-2_2005_nonspatial.pdf
http://www.fs.fed.us/foresthealth/technology/pdfs/DM_User_Guide_MAG-95-2_2005_nonspatial.pdf
Hawksworth F.G. and D. Wiens. 1996. Dwarf Mistletoes: Biology, Pathology and Systematics. Agricultural Handbook 709. Washington, DC: USDA Forest Service. 410 p.
http://www.rms.nau.edu/publications/ah_709
McCaughey, R.J. 2004. Stand Visualization System, Version 3.3. USDA Forest Service, Pacific Northwest Research Station. 141 pp.
http://www.fs.fed.us/fmsc/fvs/documents/gtrs_winsvsguide.php
Robinson, D.C.E. and B.W. Geils. 2006. Modelling dwarf mistletoe at three scales: life history, ballistics and contagion. Ecological Modelling 119:23–38. DOI: 10.1016/j.ecolmodel.2006.06.007
http://www.treesearch.fs.fed.us/pubs/24774
