Nickrent, D.L. and Musselman, L.J. 2004. Introduction to Parasitic Flowering Plants.
The Plant Health Instructor. DOI: 10.1094/PHI-I-2004-0330-01.
Updated 2016
Daniel L. Nickrent1 and Lytton J. Musselman2
1. Department of Plant Biology, Southern Illinois University
2. Department of Biological Sciences, Old Dominion University
The authors gratefully acknowledge information and ideas provided by Drs. Frank Tainter and the late Robert Eplee in the early stages of this publication.
INTRODUCTION
Fungi, nematodes, bacteria, and viruses are probably the first things that come to mind when thinking of plant pathogens. These organisms certainly do cause damage to economically important plants, but surprisingly parasitic flowering plants are also important pathogens. The purpose of this chapter is to provide an overview of the biology and evolutionary relationships of these fascinating and unusual plants and also to focus upon those that negatively affect food and fiber crops.
Most plants are autotrophs and produce their own carbon compounds through photosynthesis. Although some plants such as Indian pipe (Monotropa) (Figure 1) lack chlorophyll and appear to be parasitic, they are mycoheterotrophs (parasites of mycorrhizal fungi) and, hence, only indirectly parasitize the trees on which the mycorrhizal fungi are found. Here we define a parasitic plant as an angiosperm (flowering plant) that directly attaches to another plant via a haustorium. A haustorium is a specialized structure that forms a morphological and physiological link between the parasite and host (Figure 2) (Kuijt 1969, Yoshida et al. 2016). It is useful to make a distinction between the terms “parasite” and “pathogen.” Parasite is from the Greek para (beside) and sitos (grain or food) which literally means “beside the food”. If a plant also induces disease symptoms in a host, then it is a pathogen as well as parasite. A general term that refers to both parasites and mycotrophs that derive carbon from sources other than their own photosynthesis is heterotrophic, which simply means “different feeding.”

Figure 1. |
Figure 2. |
Types of Parasitic Plants
Parasitic plants can be categorized based on different criteria such as where they attach to the host, the degree of nutritional dependence upon the host, or whether they require a host to complete their life cycle. In terms of location on the host, two basic types can be distinguished: stem parasites and root parasites. Stem parasites occur in several families, and pathogenic members include some mistletoes and dodder (Cuscuta and
Cassytha). Root parasites are more common and occur in diverse taxonomic groups. Some of the most economically important root pathogens are in the broomrape family, Orobanchaceae. Parasitic plants may also be classified as hemiparasites or holoparasites (Figure 3). Hemiparasites contain chlorophyll when mature (hence are photosynthetic) and obtain water, with its dissolved nutrients, by connecting to the host xylem via the haustorium. Holoparasites lack chlorophyll (and are thus nonphotosynthetic) and must rely totally on the contents of the host xylem and phloem. All holoparasites require a host to complete their life cycle, thus they are obligate parasites. Root hemiparasites contain chlorophyll and some (e.g.
Triphysaria and
Odontites) complete their life cycle without hosts. But in nature, nearly all root hemiparasites are attached to host roots. Although these definitions imply absolute and discrete categories, intermediates exist such as some
Cuscuta (dodder) species that are intermediate between the hemi- and holoparasitic conditions and hemiparasitic Orobanchaceae that are intermediate between the facultative and obligate conditions.

Figure 3. |
Morphological Features
In some stem parasites, such as
Cassytha (Figure 4) and
Cuscuta (dodder) (Figure 5), the vegetative portion consists solely of a stem and scale leaves. In contrast, the casual observer may not recognize that many of the photosynthetic root hemiparasites are indeed parasites because they are green with fully formed leaves. As the degree of parasitic dependence increases (i.e. the evolution from hemiparasitism to holoparasitism), profound changes occur in the morphology of the parasitic plant. In general, holoparasites tend to have leaves reduced to scales (or absent in Hydnoraceae), succulent stems, and a primary haustorium (derived from the seedling radicle). The best examples of the evolutionary stages from hemi- to holoparasitism can be seen among various representatives of the broomrape family (Orobanchaceae, see below).

Figure 4. |

Figure 5. |
The sandalwood order (Santalales) is the most morphologically and physiologically diverse group of parasitic plants. The early diverging families in this order are autotrophic (lacking haustoria, thus nonparasitic), whereas more derived members are root parasites. In addition to mistletoes, which evolved five separate times in the order, two families (Balanophoraceae and Mystropetalaceae) are composed entirely of holoparasites.
The largest family in the sandalwood order is Loranthaceae which contains stem parasitic plants commonly known as mistletoes. The Anglo-Saxon word
misteltan derives from the Old German word "mist" for dung and "tan" meaning twig (Calder and Bernhardt 1983). This is an apropos name given that birds defecate the seeds of mistletoes onto tree branches. The connection to birds is also reflected in the Portuguese name for mistletoe:
erva de passarinho (“bird herb”). Most mistletoes are woody shrubs, often with brittle stems and leaves. However, three genera of Loranthaceae are actually root parasites and one of these (Nuytsia) of western Australia represents the first lineage to diverge in the family. Adult plants of dwarf mistletoe (Arceuthobium, Viscaceae) (Figure 6) fix very little carbon and depend heavily upon their hosts for carbohydrates. Despite this, their seedlings are photosynthetic, thus these plants cannot be considered holoparasites.

Figure 6. |
The range of flower sexual conditions (e.g. bisexual and unisexual) in parasitic plants is diverse. Dioecy (dioecious = separate male and female plants) is relatively rare in angiosperms, occurring in ca. 7% of all genera. Interestingly, dioecy is more frequent among heterotrophic plants (parasites and mycoheterotrophs) than it is among autotrophic plants. Just over 6% of autotrophic angiosperm genera are dioecious whereas over 15% of the heterotrophic genera are dioecious. This observation remains to be fully explained.
Evolutionary Development of Parasitism
What evolutionary conditions might favor the development of parasitism in plants? Root parasitism confers certain advantages, especially for annual plants. When the parasite seedling forms a haustorium, it obtains a mature, functioning root system by “assuming” the root system of its host plant. Is it energetically more cost effective for the parasite to form a haustorium on an existing root system rather than to produce one of its own? Resource allocation studies of parasites might help answer this question. Facultative hemiparasites have transpiration rates higher than their hosts, and so they are most often found in open, sunny areas. But these areas also often have dense groundcover vegetation and competition for resources in the rhizosphere is great. These conditions could have favored the evolution of root parasitism.
The stem parasitic mistletoes also exceed their hosts' transpiration rates. Given this, it is not surprising that mistletoes are most abundant in areas where access to sunlight is not limited, such as savannas and at the top of forest canopies where shading is avoided. (Vidal-Russell & Nickrent 2008) Diversification in Loranthaceae occurred during the Oligocene, a time when temperate deciduous woodlands and grasslands were displacing tropical biomes. Within the sandalwood order, each of the five cases of the evolution of aerial parasitism (mistletoes) can be traced to root parasitic ancestors. The aerial portions of woody plants certainly represented an unexploited niche that offered opportunities for colonization by such early mistletoes.
Host Interactions
The modes of host selection and specialization of parasitic plants is extraordinarily broad.
Castilleja (Figure 7) and
Cuscuta (dodder) (Figure 8) can parasitize hundreds of different hosts in diverse families; in contrast,
Epifagus virginiana (beech drops) (Figure 9) occurs only on
Fagus grandifolia (beech). The same generalization can be made about mistletoes in which some species are generalists and others specialists. Evidence exists that the generalist strategy has the greatest chance for survival over evolutionary time.

Figure 7. |

Figure 8. |

Figure 9. |
The terms
host range versus
host preference describe different aspects of the parasitic relationship. Host range refers to the total number of different species that can be parasitized. For example,
Seymeria cassioides (Figure 10) invariably attacks pines in nature, but in pot studies where a variety of angiosperms and gymnosperms are artificially inoculated, these plants are parasitized.
Host preference, referring to the choice of the most desirable host for optimal growth, typically is much narrower (e.g., Musselman and Mann, 1976).
Cuscuta (dodder) species usually have extremely broad host ranges, and can even attach to many different hosts at once. But in nature, they are found regularly on few hosts, and the parasite can often be located by first finding the preferred host.

Figure 10. |
There has been considerable research on the chemical signals between microorganisms and host roots. Accumulating evidence indicates that chemical signaling is also common in root parasites, especially germination stimulants. The seedling phase is the most vulnerable part of the life cycle because life-sustaining attachments to the host are made at this stage. In parasites with tiny (“dust”) seeds (Figure 11) with minuscule food reserves, this period is especially critical because the seedling will die in a few days without a host attachment. Parasites with larger seed reserves can survive longer. In general, parasites with tiny seeds, i.e., those less than 0.45 mm long, require a host stimulant to germinate whereas larger seeds do not.

Figure 11. |
The stem parasitic dodders (Cuscuta) provide intriguing models for host selection because they have nastic movements (in response to a host stimulus) that allow them to “forage” for (or move towards) hosts. The remarkable observation has been made that
Cuscuta pentagona uses volatile chemical cues to select hosts with the highest nutritional status (Runyon et al., 2006).
Great progress is being made in understanding host resistance responses in
Striga (witchweed) (Figure 12) and
Orobanche owing to advances in molecular biology. Many different avenues are being explored including genetically engineering the host crop plants to be resistant to the parasite as well as attenuating the virulence of the parasites themselves.

Figure 12. |
Distribution
Parasitic plants can be found in all major biomes on earth, being absent only in the coldest regions (Antarctica, North Pole).
Pedicularis dasyantha (Orobanchaceae) is found on the Svalbard archipelago of the Arctic Ocean, more than 80˚ N latitude (Musselman & Press 1995).
Nanodea muscosa (Nanodeaceae, formerly Santalaceae) is found at the other polar extreme, Tierra del Fuego of far southern South America. Tropical rainforest biomes support high plant diversity overall, however, parasitic plants make up a relatively small proportion of the flowering plants found there. Exceptions include two groups: mistletoes and holoparasites. Being stem hemiparasites, the mistletoes (mainly Loranthaceae and Viscaceae) are found high in the canopy of forest trees where sunlight is not as limited as in the understory. On the forest floor, little sunlight penetrates, hence nonphotosynthetic holoparasites such as Balanophoraceae and Rafflesiaceae can be found here. In general, parasitic plants reach their highest diversity in biomes that are not light limited such as grasslands, savannas, xeric shrublands, and even semiarid deserts. For example, the highest number of endemic genera in Orobanchaceae are found in South Africa, followed by the Southeastern United States (open, fire-maintained communities), and the Alps of Central Europe (open grasslands). The Mediterranean climate of South Africa is also the center of diversity for the root hemiparasite
Thesium (Thesiaceae, formerly Santalaceae), the most speciose genus in the sandalwood order.
Dispersal
Like many weeds, several pathogenic parasitic plants have been effectively spread by humans. Contaminated seed has spread the North American dodder (Cuscuta campestris) (Figure 13) throughout most of the world. This species of dodder is the most widespread parasitic weed in the world, and was first described (as
C. pentagona) from a major USA seaport, Norfolk, Virginia. Dodder seeds are frequently intercepted by port inspectors, confirming that ports are foci of introductions. Ballast and packing material have been implicated in the spread of
Orobanche minor (Figure 14). Another source of contamination is nursery stock and the distribution of improved cultivated varieties of crop plants. The parasite seeds enter as contaminants of crop seed or soil of potted plants. Markets have been identified as foci for the spread of
Striga (witchweed) seed to farmers in West Africa via contaminated crop seeds. In areas where improved crops have not been introduced, the likelihood of parasites being spread is lower. For example in Guinea, where subsistence grains contain little exotic germplasm,
Striga is a limited problem.

Figure 13. |

Figure 14. |
While the spread of some root parasites and
Cuscuta by humans is well documented, there are few examples of such spread for a mistletoe. One exception is
Viscum album (European mistletoe) (Figure 15) that was introduced in California as a potential crop for sale at Christmas around 1900 by Luther Burbank. An evaluation of its subsequent spread shows that it continues to expand its range at a rate of 0.35 km (0.22 miles) per year. It is interesting that the dwarf mistletoes (Arceuthobium) that are serious pathogens on conifers in the western United States have not spread to any pine species in the southeastern U.S. This probably stems from a combination of historical factors, climate, and host resistance.

Figure 15. |
Occasionally, indigenous plant parasites become serious pests when host species are planted in their habitat. There are two examples in the American South:
Seymeria cassioides (Figure 10), an annual root parasite in Orobanchaceae, and
Pyrularia pubera (Figure 16), a shrub in Cervantesiaceae (formerly Santalaceae) that is endemic to the Appalachians. Foresters were first made aware of
Seymeria in the late 1960s when slash pine (Pinus elliottii) was damaged in southern Georgia. It appears that problems with
S. cassioides are exacerbated by drought conditions. Conifers grown as Christmas trees in the mountains of West Virginia have been attacked by
P. pubera (Figure 17), thus reducing the value of the trees.

Figure 16. |

Figure 17. |
Of the approximately 1800 alien plants in California, only a few are parasites. The European mistletoe was mentioned earlier. Others include members of Orobanchaceae such as
Phelipanche ramosa, a serious parasite of tomato and other crops, and two benign hemiparasites,
Parentucellia latifolia (Figure 18) and
P. viscosa. Parasitic plants apparently spread no more quickly than non-parasitic plants. Despite this, the USDA APHIS PPQ requires permits to import or move any parasitic plant across state lines. The Federal noxious weed list contains five genera of parasitic plants that are considered to be the most serious pathogens:
Aeginetia (not yet known in U.S.),
Alectra (introduced to Puerto Rico),
Cuscuta (both introduced and native species throughout the U.S.),
Orobanche (including
Phelipanche, introduced throughout the U.S.), and
Striga (introduced to the Carolinas as well as Florida).

Figure 18. |
ECONOMICALLY IMPORTANT PARASITIC PLANTS
Cassytha, Laurel Dodder (Lauraceae-Laurel Family)
Cuscuta, Dodder (Convolvulaceae-Morning Glory Family)
Orobanchaceae (Broomrape Family)
Striga (Witchweeds-Broomrape Family)
Orobanche (Broomrapes-Broomrape Family)
Sandalwood Parasites
Loranthaceae (Showy Mistletoe Family)
Santalaceae (Sandalwood Family)
Viscaceae (Christmas Mistletoe Family)
Cassytha, Laurel Dodder (Lauraceae-Laurel Family)
Cassytha is a high climbing, parasitic vine with green or orange stems that bears a remarkable resemblance to
Cuscuta (dodder).
Cassytha is regularly mistaken for dodder, even by experienced plant scientists. The similarity between these two unrelated genera is one of the most striking parallelisms among the flowering plants. Additional features common to both
Cuscuta and
Cassytha are hard seeds that require scarification (breaking or scratching of the seed coat, but no host stimulant) and simultaneous attachment of a single parasite to several diverse hosts.
Cassytha is the only parasitic member of the otherwise autotrophic Laurel family. The host range of
Cassytha is broad. Considering its vigor (Figure 19) and wide distribution, it is remarkable that it is only occasionally reported as a significant pest usually on tree crops including mango (Mangifera indica) and avocado (Persea americana). Most species occur in Australia, but
C. filiformis is pantropical and is also abundant in southern Florida. Because the species are superficially similar, a new introduced species could easily be overlooked.

Figure 19. |
Cuscuta, Dodder (Convolvulaceae-Morning Glory Family)
Species of
Cuscuta are among the best known of all parasitic plants. The biology and control of dodders was reviewed in Dawson et al. (1994).
Cuscuta has a broad host range, although monocots are less preferred (Figure 13). The genus
Cuscuta contains three subgenera. Members of the subgenus
Monogynella are robust vines that may attack and kill fruit trees, while species in the subgenus
Cuscuta are more delicate in structure and favor herbaceous hosts, as do species of the entirely New World subgenus,
Grammica.

Figure 13. |
Dodders may be the most important parasitic weeds of legumes in temperate regions. Of particular importance is
C. campestris (Figure 5) on alfalfa (Medicago sativa) with especially significant impact on alfalfa grown for seed. The alfalfa and dodder seeds are similar in size, and so the parasite is spread with the host. The wide range of hosts attacked by dodders is reviewed in Dawson et al. (1994). The most effective means of control is seed sanitation. Because the surface of dodder seeds is minutely roughened, dodder seeds stick to felt rollers while alfalfa seeds pass over. Dawson et al. (1994) also reviewed several herbicide treatments that are directed at the newly germinated seeds of dodder.

Figure 5. |
Orobanchaceae (Broomrape Family)
This family includes the largest number of genera (over 90) and species (ca. 1900) of all the families of parasitic flowering plants. In the past, the hemiparasitic members of this family were classified as part of Scrophulariaceae (the figwort family) while the holoparasitic members were included in Orobanchaceae (the broomrape family). Molecular phylogenetic studies have shown that all of these parasitic plants are monophyletic (i.e. all share a common ancestor) and are thus classified in Orobanchaceae. The two most economically important genera,
Striga (witchweed) and
Orobanche (broomrape), are discussed below, with particular attention paid to their life cycle similarities and differences.
Striga (witchweeds)
Witchweeds (Striga spp.) have a greater impact on humans worldwide than any other parasitic plant because their hosts are subsistence crops grown widely in Africa and Asia. Such crops include maize, sorghum, pearl millet, finger millet, rice, and sugar cane as well as legume crops such as cowpea and ground nut. The name “witchweed” derives from the effect these parasites have on their host in which most damage occurs before the parasite is visible above ground. This “bewitching” behavior is also reflected in the Latin name, which means “hag” or “witch.”
Striga is an obligate hemiparasite that reaches its greatest diversity in the grasslands of Africa, although it also occurs in India, eastern Asia, and Australia. Two species,
S. asiatica (Figure 12) and
S. hermonthica (Figure 20), cause the most damage to crops worldwide with often devastating yield reduction in the African Sahel.
Striga species have complex life cycles involving several discrete steps: seed dispersal, after ripening, seed conditioning, germination, haustorial induction, attachment, penetration, seedling development, emergence, flowering, and fruiting. The following summary is based on reviews in Sauerborn (1991), Parker and Riches (1993), and Mohamed et al. (2001).

Figure 12. |

Figure 20. |
Striga produces thousands of “dust” seeds (Figure 11) per capsule that are shed when the rains stop at the end of the growing season. Movement of seeds in nature is usually attributed to wind and rain, facilitated by the distinct sculpturing on the seed. Seeds are dormant for several months before they will respond to chemicals exuded by the host; this period is referred to as “after ripening.” Conditioning occurs when two environmental factors are present: suitable soil temperatures in the range of 25-35°C (77-95° F) and moisture content near 100%. During conditioning, the seed imbibes water, and after conditioning, it is able to respond to chemical signals (Figure 21) from the host roots. The signals indicate both the type of host and the distance to the host. Recent work has shown that host roots exude plant growth regulators called strigolactones, a group of sesquiterpene lactones. These molecules are also known to stimulate mycorrhizal fungi colonization as well as regulate shoot and root branch formation in nonparasitic plants. Orobanchaceae have evolved the ability to recognize these chemicals as germination stimulants. Little is known about after-ripening though the role of reduction of abscisic acid levels has been suggested. Perhaps after-ripening leads to a down regulation of endogenous strigolactones making the witchweed seeds sensitive to external, host-derived strigolactones. Germination in obligate root parasites is usually cryptocotylar, that is, the cotyledons remain within the seed coat when the delicate radicle emerges. The subterranean communication between host and parasite has been intensely studied in the past decade. These complex processes are a kind of botanical “eavesdropping” with intricate molecular pirouetting. After germination, the radicle of the parasite stimulates host peroxidases and laccases that oxidize the lignin of host cell walls producing 2, 4-dimethoxybenzoquinone (DMBQ). Through redox cycling of DMBQ, signaling occurs that initiates haustorium development on the parasite radicle. At this stage, the
Striga radicle tip begins to produce structures that resemble root hairs that extend to contact the host root (Figure 22). These hairs “glue” the
Striga root to the host (Figure 23). Similar hairs are produced by some other members of Orobanchaceae. If the host is suitable, the haustorium penetrates and forms a link with the host vascular system; this is the penetration stage.

Figure 11. |
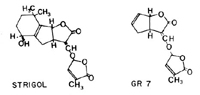
Figure 21. |
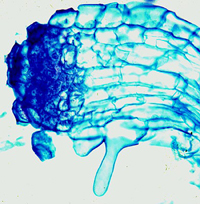
Figure 22. |
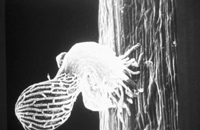
Figure 23. |
As the parasite becomes established and matures, the distinctive seedling of
Striga is formed (Figure 24)(Figure 25). It is subterranean, lacks chlorophyll, possesses scale leaves and produces abundant adventitious roots from which additional (lateral) haustoria can arise. The seedling exerts a powerful influence on the growth-regulatory systems of the host by altering hormone balances and stimulating root production. Significant host damage can occur at this stage. Parasite shoots draw on host resources and elongate upwards through the soil to reach the surface. After emergence, chlorophyll develops, flowers form, and the life cycle is completed when the seeds are produced.
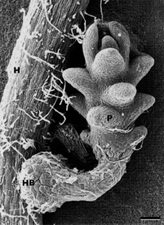
Figure 24. |

Figure 25. |
Why have so few
Striga species become serious pests? One hypothesis is that the widespread
S. hermonthica and
S. asiatica are true agrestals, that is, plants associated only with agroecosystems (Mohamed et al. 2001). They suggest that these crop pathogens are spread with their hosts, are more abundant in crops than native grasslands, and have evolved from native species. Two observations support this. First,
S. hermonthica and
S. asiatica have never been reported in native grasslands in Africa. Second, in areas where there has been little introduction of improved grain varieties,
Striga is a limited problem, as noted earlier for Guinea. A similar situation exists in Togo where
S. asiatica became a serious problem only after improved varieties of maize began to be widely grown.
When
Striga was identified in the Carolinas, USA in 1956, federal and state action was initiated in response to this threat. Dedicated research facilities were established to conduct basic and applied research to gain an understanding of witchweed and to develop methodology to eradicate the infestation. Early research revealed that
S. asiatica could be controlled in maize by using a non-volatile form of the selective phenoxy herbicide 2,4-D. This method stopped the reproduction of the
Striga, but did not prevent early damage to the crop. Motorized high clearance sprayers and backpack sprayers were used, but the magnitude of the infestation (thousands of hectares) called for more effective methods. Moreover, the method could not be used to control witchweed growing on weedy grasses in broadleaf crops such as cotton, soybeans and vegetables because these plants are susceptible to this herbicide.
A major problem in witchweed control is the persistence of the microscopic seeds in the soil. Research was conducted to try to identify the seed germination stimulant. A breakthrough occurred when it was found that ethylene gas could induce
Striga seed germination. New equipment and application methodologies to introduce ethylene gas into the soil were developed (Figure 26). Under proper soil conditions, the injection of 1.5 kilograms per hectare of ethylene stimulates the suicidal germination of up to 90% of the viable
Striga seed bank in a growing season. The witchweed control and containment program in North and South Carolina is likely the most successful example of controlling of a parasitic weed. From its discovery in 1956,
Striga reached its greatest distribution in 1979 when 380,000 acres were infested. By 2015 only 1,100 infested acres remained; however, it still remains a potential threat to agriculture in states as far north as Pennsylvania and west to Iowa and much of Texas, thus continued monitoring is required.

Figure 26. |
Orobanche (Broomrapes-Broomrape Family)
The genus
Orobanche, as currently classified, contains ca. 78 species of obligate root holoparasites from the Old World. It is sometimes split into two genera,
Orobanche and
Phelipanche based on technical characters. Species of both genera are known by the English name broomrape because they were thought to grow as tubers (“rapum”) from brooms (the common name for the legume
Cytisus). The genus reaches its greatest diversity in Mediterranean climates and in Western Asia. All of the economically important pathogens are Old World species. New World species, previously classified as
Orobanche such as
O. fasciculata and
O. uniflora, have been shown via molecular phylogenetic studies to belong to the genus
Aphyllon. Moreover, some Old World species have also been segregated from
Orobanche (genera
Boulardia,
Myzorrhiza, and
Phelipanche).
Major crop hosts for
Orobanche are legumes, solanaceous crops (eggplant, tomato, tobacco, potato but not
Capsicum peppers), umbels (carrot, parsley, celery), cole crops (cabbage, cauliflower), lettuce, and sunflower. Control is difficult because of seed longevity in the soil (more than five decades), small seed size (less than the width of a human hair), fecundity (thousands of seeds per plant), and a subterranean phase (seeds germinate beneath the soil and parasitize the host before they emerge and becoming evident). Broomrapes have their greatest impact in the Balkans, the Nile Valley, Central Asia, southern India, and Nepal. Damage varies with level of infestation, and total crop failures have occurred in some cases.
There have been numerous studies of the host range of
Orobanche species. It has been shown that
O. ramosa (=
Phelipanche ramosa) (Figure 27)(Figure 28) can parasitize plants from 11 different dicot families, in fact, more different hosts than any other broomrape. Major agronomically important hosts include solanaceous crops, cabbage, cauliflower, hemp, carrots, lettuce, and some legumes. The related species
O. aegyptiaca (=
Phelipanche aegyptiaca) causes especially severe damage to melons in Central Asia where broomrape not only reduces the yield and weakens the melons, but also induces the production of a toxin within the melons that renders them unmarketable.

Figure 27. |

Figure 28. |
Evaluation of host range for parasitic plants is complicated by the fact that crops parasitized in pots may not be parasitized in the field. For example, there are no reports of field parasitism of soybean by any broomrapes, although soybean was parasitized by
O. crenata in pots. Nonetheless, pot experiments are valuable because potential hosts can be identified and host specific strains of the parasites can be determined.
Germination requirements for
Orobanche are different from those of
Striga. In general,
Orobanche is a parasite of colder climates, so germination temperatures range from 10 to 20°C (50 to 68°F), alternating with temperatures as low as 5°C (41°F). This may explain why
O. ramosa is a problem in the Nile Valley of Sudan only in the winter, and may also explain why
O. cernua attacks tobacco in the winter in India, but is not a problem on sunflower (Helianthus annuus) grown in the same region in the summer. Unlike
Striga,
Orobanche seedlings produce roots that are geotropically neutral, that is, they do not grow downward in response to gravity.
Orobanche also differs from
Striga in the way the host root system is affected.
Orobanche noticeably weakens the roots of its host. An exception is
O. cernua, which often forms a large, single haustorium on a strengthened host root. The role of ethylene, important for witchweed germination and a useful control for that parasite, is unclear in
Orobanche germination.
The longest documented viability of
Orobanche seeds in the soil was a case in Bulgaria. In 1956 some of the tobacco fields near the Tobacco Research Institute near Plovdiv were replanted in grapes due to severe broomrape infestation. In 1991 the vineyards were removed and tobacco replanted. Large numbers of
O. ramosa emerged, presumably from seeds that had been dormant since the last time tobacco had been grown!
Orobanche ramosa has been introduced to California where it has been a problem on tomatoes (Figure 29). Despite a concerted effort to eradicate it, the parasite still persists. It is likely that seeds of the parasite were introduced with the crop, either through contaminated plants or contaminated tomato seeds. More recently it has been found at numerous sites in eastern Virginia. The relatively recent infestations of tomato in Chile should serve as a warning that we can expect
O. ramosa to establish itself wherever suitable hosts are found.

Figure 29. |
Orobanche cernua (Figure 30) is widespread in Eastern Europe and the Middle East with heavy infestations in southern India and scattered occurrences in North Africa, China, and southern Europe. Its primary hosts are solanaceous crops and sunflower. The parasite of sunflower is taxonomically classified as
O. cernua var.
cumana or
O. cumana. Sunflower is the most important oil crop in parts of eastern Europe, and
O. cernua is a major constraint on production, especially in Bulgaria where sunflower oil is very important (Figure 31). Infected plants are stunted and have smaller heads with aborted fruits and poorer oil quality. Between 1947 and 1950, the broomrape problem became so severe in Bulgaria that it threatened the continued cultivation of sunflower. New, resistant varieties were introduced from the Soviet Union, but host resistance apparently selected for virulence in broomrape, with a resultant rapid loss of the resistance. Nine “races” of the parasite were documented in Bulgaria with new races recently reported from Spain. Tobacco can also be severely damaged by
O. cernua. However, tobacco is not attacked in Bulgaria by
O. cernua, even in fields adjacent to heavily infested sunflower fields. The situation is reversed in India, where tobacco is severely damaged and sunflower has not been attacked. This difference in host preference, studied for years by European and Russian plant breeders, supports the concept that there is genetic differentiation that might be recognized taxonomically (i.e.
O. cernua vs.
O. cumana). These data should be re-examined from a modern genetic perspective. More awareness of this pathogen is needed, considering the acreage devoted to sunflower in the United States.

Figure 30. |

Figure 31. |
The last of the major broomrapes to consider is
O. crenata (Figure 32). Major hosts include broadbeans, lentils, forage legumes, carrots, parsley, and celery. It is restricted to Europe, the Middle East and North Africa. A report of
O. crenata in Finland attests to the cold hardiness of this species, which has the northernmost range of the agronomically important broomrapes. Damage is severe and commodities from infected areas should be carefully monitored.

Figure 32. |
Three other genera of Orobanchaceae,
Aeginetia Alectra, and
Christisonia, may be considered minor problems on monocots, unusual hosts for most broomrapes.
Aeginetia indica (Figure 33) can be damaging on cereals and there are scattered reports of
Christisonia scortechinii damaging sugarcane in the Philippines.
Alectra is an increasing problem on cowpea and peanut (groundnut) in central Africa.

Figure 33. |
Sandalwood Parasites
The Sandalwood order (Santalales) has the widest range of trophic modes among all parasitic plants, including autotrophs, root hemiparasites, stem hemiparasites, and root holoparasites. The mistletoe habit has evolved independently five times in this order, thus mistletoe refers to the plant habit (stem parasite), not a taxonomic group. The order contains 12 genera (57 species) of autotrophs, 150 genera (2370 species) of hemiparasites, and 17 genera (42 species) of holoparasites. These plants are distributed worldwide with greatest diversity in the tropics. In terms of impacting human activity, four families deserve mention: Loranthaceae, Thesiaceae, Cervantesiaceae, and Viscaceae.
Loranthaceae (Showy Mistletoe Family)
This large, mainly tropical, family is composed of 76 genera and over 1000 species. The flowers are variable in size and shape, but many members have large showy flowers (Figure 34)(Figure 35) that are bird-pollinated. Indeed, the co-evolutionary relationship with birds has reached a high level in this family, as evidenced also by the seed dispersal mechanism. In Australia, birds of the genus
Dicaeum have tongues that are specifically modified to sip nectar from mistletoe flowers and digestive canals that pass the viscid seeds in a remarkably short period of time. Many other mistletoe-animal interactions occur, and Watson (2001) has proposed that mistletoes function as keystone resources in many ecosystems, i.e. they are important ecological components that positively affect diversity in these habitats.

Figure 34. |
At least 30 genera of mistletoes in the Loranthaceae occur on introduced or cultivated trees, and the following have been reported to be particularly damaging:
Tapinanthus bangwensis (Figure 35) on cacao (Theobroma cacao) in Africa,
Dendrophthoe pentandra (Figure 36) on kapok (Ceiba pentandra) in Java,
Passovia pyrifolia (Figure 37) on teak (Tectona grandis) in Trinidad, and
Oryctanthus occidentalis. (Figure 38) on cacao in Costa Rica. Four genera of Loranthaceae (Agelanthus,
Englerina,
Globimetula, and
Tapinanthus) are particularly damaging to the shea butter or karité tree (Vitellaria paradoxa, Sapotaceae) in Burkina Faso. Of the 16,000 trees examined in one study, 95% were parasitized by one or more of these mistletoes. Management of loranthaceous mistletoes generally involves pruning the mistletoe and/or the host branch.

Figure 35. |

Figure 36. |

Figure 37. |

Figure 38. |
A discussion of a typical member of Loranthaceae (“loranth” here) follows, beginning with flowering. Birds pollinate the large-flowered Old and New World species, whereas insects are the pollen vectors for small-flowered species. Some loranths have bisexual flowers, whereas others are dioecious or monoecious with unisexual flowers. Loranthaceae are unusual among angiosperms in having an “aggressive” embryo sac which actually grows out of the ovule into the ovary and even into the style in some species. After fertilization, the embryo and seed begin to form within the inferior ovary. The fruit (Figure 39) that develops is a single-seeded berry (or drupe, but without a stony endocarp), and the enclosed seed (Figure 40) is surrounded by sticky viscin. The viscin, composed of cellulosic strands surrounded by mucilaginous pectic material, attaches the seed to the host plant after dispersal. Unlike Orobanchaceae, loranth seeds do not require a host germination stimulant and will germinate spontaneously; however, establishment only occurs on living hosts. Emerging from the photosynthetic endosperm, the seedling radicle (or hypocotyl) is negatively phototropic and thus grows towards a dark surface (often the host branch) (Figure 41). The first attachment structure formed is called a holdfast (Figure 42), and the cotyledons either remain within the endosperm as absorptive structures (e.g.
Loranthus) or emerge and expand (e.g.
Psittacanthus) (Figure 43). The haustorium forms from the holdfast, eventually connecting to the host xylem. The first aerial shoots of the mistletoe usually form from the epicotyl and, in some species, epicortical roots (Figure 44) extend from the haustorium along the host branch. These epicortical roots form new haustoria and shoots, thus allowing lateral spread within the host branches (Figure 45). When in contact with the host cambium, the loranth haustorium induces the formation of additional wood that enlarges in fluted columns, eventually forming a placenta-like saddle. This structure is called a woodrose (Figure 46). When the mistletoe haustorial tissue (composed mainly of parenchyma) decays, the woodrose remains and is often used for decorative art objects (Figure 47).

Figure 39. |

Figure 40. |

Figure 41. |

Figure 42. |

Figure 43. |

Figure 44. |

Figure 45. |

Figure 46. |

Figure 47. |
Sandalwood Family Relatives
The root hemiparasitic Santalaceae that includes the sandalwood of commerce (Santalum spp.) traditionally included nearly 40 genera. Molecular phylogenetic studies resulted in a reclassification that recognizes additional families, thus reducing Santalaceae to 11 genera. Although this newly circumscribed family contains three genera of mistletoes, none are economically significant from a plant pathology perspective. Indeed, the opposite is true for
Santalum album, which is extensively cultivated because it is the source for santalol, a compound used in the preparation of perfumes, cosmetics, and medicine. Two families that have pathogenic members are Thesiaceae and Cervantesiaceae. Although
Thesium contains weedy species, only a few cause significant damage. In Cervantesiaceae,
Pyrularia pubera (Figure 16) can be a pathogen of trees under some circumstances in the eastern U.S. (as mentioned above). It is of interest that
Okoubaka aubrevillei of West Africa, also a member of this family, can inflict severe damage on surrounding vegetation.

Figure 16. |
Viscaceae (Christmas Mistletoe Family)
The mistletoe family Viscaceae is small in terms of the number of genera (seven), but contains a large number of species (578). Most of these species occur in two genera:
Viscum (of the Old World) and
Phoradendron (of the New World). The name Viscaceae derives from a feature of the seeds - the viscin - that forms a sticky layer on its outer surface (Figure 48), (Figure 49) which attaches the seeds to the host branch. These are the mistletoes most often recognized by people from temperate parts of the world because the green leafy shoots with white berries (Figure 50) often festoon doorways around Christmas time. For more about the folklore associated with mistletoe, see the Feature Story by Tainter (December, 2002) at:
http://www.apsnet.org/publications/apsnetfeatures/pages/mistletoe.aspx.
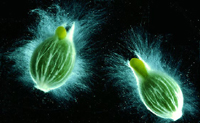
Figure 48. |

Figure 49. |

Figure 50. |
Mistletoes in Viscaceae impact both positively and negatively on human activities. In addition to Christmas decoration,
Viscum album (Figure 15) is used medicinally, for example to treat various forms of cancer. Although the efficacy of some of these practices is questionable, there is growing scientific evidence of therapeutic activity. Notably, recombinant mistletoe lectin (rML) has been used to treat ovarian cancer. The other major compounds extracted from
Viscum are the thionins, termed viscotoxins (VT) that not only have immunomodulatory effects, but are also strong cytotoxins. These cytotoxins are present in mistletoe berries, and thus pose a safety risk for small children who may ingest them.

Figure 15. |
Certainly the greatest economic impact on human activity is caused by mistletoes in the genus
Arceuthobium (Figure 6). Termed “dwarf mistletoes” because some species are diminutive, their damaging effects upon commercially important forest trees in North America are enormous: 11.3 million cubic meters of wood is lost annually (Hawksworth and Wiens 1996). Although “leafy mistletoe” such as
Phoradendron and
Viscum can also cause damage to host trees, particularly in urban environments, it is not on the same scale as
Arceuthobium and commercial species are seldom impacted. The leafy mistletoes occur on both hardwood and coniferous hosts, whereas
Arceuthobium is known only from conifer hosts (families Pinaceae and Cupressaceae).

Figure 6. |
The life cycle of viscaceous mistletoes is similar to that described above for Loranthaceae, but with some exceptions. Pollination is generally effected by insects and wind, and the flowers in this family are very small. The monoecious or dioecious plants bear unisexual flowers on spikes or cymes (Figure 51) (Figure 52) (Figure 53). In several genera, leaves are reduced to scales. The haustorium of Viscaceae never forms epicortical roots, but instead forms a complex internal organ called the endophyte. This structure is composed of portions that run parallel to the host branch axis within the cortex, hence they are called cortical strands. Other tissues called sinkers descend perpendicularly from the cortical strands into the host xylem (Figure 54). Mistletoes in Viscaceae are water hemiparasites, hence they manufacture at least some of their own food through photosynthesis. Indeed, it has been documented that leafy mistletoes such as
Phoradendron actually translocate photosynthates back into the host during the winter when host leaves are absent. In contrast,
Arceuthobium (dwarf mistletoe) produces very little of its own food as an adult plant.

Figure 51. |

Figure 52. |

Figure 53. |
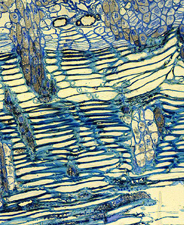
Figure 54. |
Complex types of haustoria are found in
Arceuthobium. In some cases the mistletoe haustorium is localized and the only effect on the host is a smaller branch distal to the swollen infection. In other cases, a structure called a witches’ broom is induced by the mistletoe haustorium that is composed of a dense group of host branches. Two types of witches’ brooms are known: nonsystemic (Figure 55) where the mistletoe endophyte does not enter the witches' broom; and systemic (Figure 56), where the endophyte is present, sometimes even in the host branch apical meristem. When the host cells divide by mitosis, the parasite endophyte cells also divide in a coordinated fashion. Systemic witches’ brooms, as seen in
A. douglasii and
A. pusillum, are remarkable examples of the extremely intimate cellular relationship that has evolved between host and parasite.

Figure 55. |

Figure 56. |
In
Viscum,
Phoradendron, and most of the other genera of Viscaceae, seed dispersal is mediated by birds. In all but one species of
Arceuthobium, birds are not the primary seed vectors. Instead the fruits have an explosive dehiscence mechanism for dispersal. The fruit walls are very elastic, and turgor pressure develops as the fruit ripens. A layer of cells that connects the fruit to the pedicel begins to weaken and, with the slightest disturbance, the fruit separates forcefully from the pedicel (Figure 57). This hydrostatic mechanism explosively expels the single seed at a rate of 27 meters (yards) per second (Figure 58), and the seed can travel up to 16 meters (yards) before sticking to a host surface (Figure 59). Moisture from dew or rain causes the viscin on the seed to swell, and the seed may then slide to the base of the needle fascicle where germination ensues (Figure 60). Although many seeds are wasted in this process, one plant may produce hundreds of fruits and seeds, thus many are successfully established on young tree branches. The seeds can only penetrate younger host tissue because there the bark is thin.

Figure 57. |
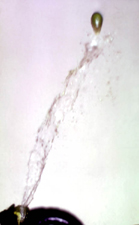
Figure 58. |

Figure 59. |

Figure 60. |
As with loranths, the seedling radicle forms a holdfast when it encounters an obstruction on the host branch, and the haustorium penetrates the host from within the holdfast. Unlike most Loranthaceae and Viscaceae, the shoots of
Arceuthobium (dwarf mistletoes) are not formed from an epicotyl; once the endophyte is inside the host, the radicle (hypocotyl), cotyledons, and endosperm wither and die. The endophyte then develops within the host and aerial shoots form as much as a year later adventitiously from the cortical strands (Figure 61). In nature, various dwarf mistletoe species may require from three to ten years to go from seed to seed.

Figure 61. |
Controlling Dwarf Mistletoes - a Continuing Challenge
Dwarf mistletoes are often a serious threat in extensively managed forests, hence, control measures must be economical. Although there is no relationship between presence of dwarf mistletoe and site quality, once dwarf mistletoe invades a site, infected trees growing on a poor site are invariably more seriously affected than are infected trees growing on a good site. Thus, an effective form of control is to determine site quality before dwarf mistletoe has become a problem. On good sites, selective removal of infected trees during pre-commercial and commercial thinnings might be effective enough to reduce almost all losses. On poor sites, heavy dwarf mistletoe infestation might dictate early harvest or even abandonment of the stand for commercial purposes. In high-value stands such as seed orchards or recreation areas, severely infected trees should be felled. For valuable trees, pruning of infected branches can be effective if more than half the crown will remain after the infected branches are removed. The USDA Forest Service has developed a computer program that is used by forest managers to reduce losses due to dwarf mistletoe.
Natural wildfires effectively eradicated local infestations of dwarf mistletoe in the prehistoric past, and so prescribed fire has been investigated as a possible control agent. Fire has been most effective with
Arceuthobium pusillum in the eastern spruce forests because this species spreads locally to form discrete infection centers with a distinct boundary between diseased and healthy trees. The use of fire has been less effective in western North American forests because here dwarf mistletoes do not generally form discrete infection centers. Instead, economic losses attributed to dwarf mistletoes are effectively minimized with silvicultural treatments that recognize stand site quality and then reduce the incidence of infected trees by thinning. In some situations, resistant tree species can be planted to replace a susceptible species presently on the site. For example, ponderosa pine can be replaced by Douglas fir, or by hardwoods such as aspen or cottonwood.
Chemical control through the use of herbicides has limited success, mainly because it is expensive and difficult to find chemical agents that affect only the mistletoe and not the host. An environmentally safe chemical, ethephon, effectively controls dwarf mistletoe in some situations. When applied in summer, the dwarf mistletoe shoots will dry up and fall off before seeds can be produced. The chemical will not affect the endophytic system, so the infected trees will need to be resprayed every few years when new dwarf mistletoe shoots appear. This technique is only useful where high-value trees are involved.
Various means of biological control have been investigated, including insects and fungi. These organisms, however, have coevolved with
Arceuthobium, hence it is unlikely they can be used to dramatically reduce the size of mistletoe populations.
Arceuthobium is native to North America, Asia, Europe, and Africa - the only mistletoe that occurs naturally in both the Old and New Worlds. These plants have been coevolving with their hosts for millions of years, so it is probably unrealistic to achieve total elimination. Moreover, dwarf mistletoe witches' brooms provide roosts and nesting sites for many birds and animals (including the Northern Spotted Owl), thus there is a growing tendency to take an enlightened view of the role these parasites play in the complex forest ecosystem (Watson 2001), and to modify management practices with this view in mind.
CONCLUSION
Although 277 genera and 4750 species of flowering plants are parasitic, only about 25 genera negatively impact host plants cultivated by humans and are thus considered pathogens (Table 1). Among these, four genera are the most damaging pests:
Striga,
Orobanche (including
Phelipanche),
Cuscuta, and
Arceuthobium. The impact of
Striga is greatest on farmers growing subsistence crops such as maize, sorghum, pearl millet, and cowpea in Africa and Asia. The broomrapes are pests worldwide but are particularly damaging to legumes and solanaceous crops in the Middle East.
Striga and
Orobanche produce thousands of tiny seeds that persist in the soil, thus control has proven difficult. The dwarf mistletoes represent one of the major disease organisms affecting commercially harvested coniferous trees. As native components of the ecosystem that have coevolved with their hosts for millions of years, management rather than eradication is the only rational approach. Understanding the complex and fascinating biology of parasitic flowering plants will require continued research by those engaged in both basic and applied scientific disciplines.
Table 1
Selected Pathogenic* Parasitic Angiosperms |
|
Parasite |
Hosts |
Distribution |
Convolvulaceae
dodder |
|
Cuscuta campestris Is the most widely distributed of about ten species that attack crops. | diverse crops, specially legumes | Worldwide |
|
Lauraceae |
|
Cassytha filiformis | Woody plants | Pantropical |
|
Orobanchaceae |
|
Aeginetia indica | sugarcane (Saccharum) | India, Philippines, Indonesia |
|
Alectra vogellii A few other species have been reported as pests of sunflower and tobacco. | cowpea (Vigna unguiculata) | Africa |
|
Christisonia wightii | sugarcane (Saccharum) | Philippines |
|
Broomrapes |
|
Orobanche ramosa | Various crops, especially Solanaceae | Most serious in Mediterranean climates but widely spread. |
|
O. crenata | Mainly legumes; carrots | Most serious in Mediterranean Climates. |
|
O. minor | legumes, tobacco, carrots, some other crops | Widely spread in temperate regions. |
|
Rhamphicarpa fistulosa | peanut (Arachis), rice (Oryza) | Africa |
|
Seymeria cassioides | pine (Pinus spp.) | Southern USA |
|
Witchweeds |
|
Striga hermonthica | grains | Semi-arid Africa |
|
S. asiatica | grains | Africa, introduced to North and South Carolina |
|
S. gesnerioides | legumes | Africa, introduced to Florida |
|
Loranthaceae |
|
Agelanthus spp. | shea butter or karité (Vitellaria) | Africa |
|
Amyema spp. |
Eucalyptus | Australia |
|
Tapinanthus bangwensis | cacao (Theobroma), etc. | Africa |
|
Dendrophthoe spp. | kapok (Ceiba), etc. | Asia |
|
Phthirusa spp. | rubber (Hevea), teak (Tectona) | Central & South America |
|
Psittacanthus spp. |
Citrus | Mexico |
|
Struthanthus spp. |
Coffea, Citrus, etc. | Central & South America |
|
Santalaceae |
|
Acanthosyris pauloalvimii | cacao (Theobroma) | Brazil |
|
Exocarpos spp. |
Eucalyptus | Australia |
|
Osyris alba | grape (Vitis) | Yugoslavia |
|
Pyrularia pubera | fir
(Abies fraseri) | West Virginia, USA |
|
Thesium spp. | sugarcane (Saccharum) barley (Hordeum), etc. | Australia, Spain, Africa, USA |
Viscaceae
dwarf mistletoes |
|
Arceuthobium spp. | Pinaceae (New World)
& Cupressaceae (Old World) | North America, Europe, Asia, Africa |
| "Christmas" mistletoes |
|
Dendrophthora poeppigii | rubber (Hevea) | Brazil |
|
Phoradendron spp. | various trees | North, Central and South America |
|
Viscum spp. | various trees | Europe, Africa, Australia, Asia |
| * Pathogenic defined as negatively impacting a host plant that is cultivated by humans. |
REFERENCES
Calder, D. M. and P. Bernhardt. 1983. The Biology of Mistletoes. Academic Press, New York.
Dawson, J. H., L. J. Musselman, P. Wolswinkel, and I. Dörr. 1994. Biology and control of Cuscuta. Rev. Weed Sci. 6: 265-317.
Hawksworth, F. G., and D. Wiens. 1996. Dwarf mistletoes: Biology, pathology, and systematics. USDA Forest Service, Washington D.C.
Heide-Jørgensen HS. 2008. Parasitic Flowering Plants. Leiden, Boston: Koninklijke Brill NV.
Joel, D. M., J. G. Gressel, and L. J. Musselman. 2013. Parasitic Orobanchaceae: Parasitic Mechanisms and Control Strategies. Berlin: Springer.
Kuijt, J. 1969. The Biology of Parasitic Flowering Plants. University of California Press, Berkeley, CA.
Mathiasen, R. M., D. L. Nickrent, D. C. Shaw, and D. M. Watson. 2008. Mistletoes: pathology, systematics, ecology, and management. Plant Disease 92: 988-1006.
Musselman, L. J. and W. F. Mann. 1978. Root parasites of southern forests. U.S. Dep. Agric. For. Serv. Gen. Tech. Bull. SO-20,76 p. South. For. Exp. Stn., New Orleans, LA.
Musselman, L. J., and M. C. Press. 1995. Introduction to parasitic plants. Pages 1-13 in M. C. Press and J. D. Graves, eds.
Parasitic Plants. Chapman and Hall, London.
Nickrent, D. L. V. Malécot, R. Vidal-Russell, and J. P. Der. 2010. A revised classification of Santalales. Taxon 59: 538-558.
Press, M. C., and J. D. Graves. 1995. Parasitic Plants. Chapman and Hall, London.
Sauerborn, J. 1991. Parasitic Flowering Plants. Ecology and Management. Verlag Josef Margraf, Weikersheim.
Parker, C. and C. R. Riches. 1993. Parasitic Weeds of the World. CABI, Oxford.
Runyon, J. B., M. C. Mescher, and C. M. De Moraes. 2006. Volatile chemical cues guide host location and host selection by parasitic plants. Science 133: 1964-1967.
Mohamed, K. I., L. J. Musselman, and C. R. Riches. 2001. The Genus Striga (Scrophulariaceae) in Africa. Ann. Mo. Bot. Gard. 88: 60-103.
Vidal-Russell, R. and D. L. Nickrent. 2008. The first mistletoes: origins of aerial parasitism in Santalales. Molecular Phylogenetics and Evolution 47: 523-537.
Watson, D. M. 2001. Mistletoe - a keystone resource in forests and woodlands worldwide. Ann. Rev. Ecol. Syst. 32: 219-249.
Yoshida, S., Cui, S., Ichihashi, Y., and Shirasu, K. 2016. The haustorium, a specialized invasive organ in parasitic plants. Annual Review of Plant Biology 67:643-667.
WEBSITES
The Parasitic Plant Connection:
http://www.parasiticplants.siu.edu/
International Parasitic Plant Society:
http://www.parasiticplants.org/
Haustorium: Parasitic Plant Newsletter:
http://www.odu.edu/~lmusselm/haustorium/index.shtml
Ecology and Management of Parasitic Weeds:
https://www.uni-hohenheim.de/www380/380b/science/supraregional/start.htm
