Amy Y. Rossman and Mary E. Palm, USDA Agricultural Research Service, USDA Animal and Plant Health Inspection Service, Systematic Botany & Mycology Laboratory, Beltsville, Maryland 20705
Adapted with permission of Research Information Ltd. from Outlooks on Pest Management 17: 217-219. 2006. - see
www.pestoutlook.com.
Introduction
Plant diseases result in billions of dollars in damage to agricultural crops each year. One of the groups of organisms that cause many serious plant diseases has long been known as the Oomycota or oomycetes, traditionally classified in the phycomycetes or “lower fungi.” The phycomycetes are an informal group that, in addition to the Oomycota, has historically included such diverse organisms as the slime molds, chytrids, zygomycetes or bread molds, and arbuscular mycorrhizae. The “higher fungi” have traditionally included the ascomycetes and the basidiomycetes. Examples of ascomycetes, or sac fungi, include the discomycetes (e.g. morels), pyrenomycetes (including the cause of chestnut blight), deuteromycetes or imperfect fungi, and yeasts. The basidiomycetes, or club fungi, include rust fungi, smut fungi, mushrooms, gasteromycetes, polypores, and jelly fungi. However, over the past three decades knowledge about relationships among groups of fungi has increased greatly such that we now realize that many of the traditional morphology-based groupings no longer reflect phylogenetic relationships.
The Oomycota or Peronosporomycota consist of more than 800 species that may be saprobic or parasitic on terrestrial or aquatic plants and animals. One member of the Oomycota has greatly influenced history, namely
Phytophthora infestans, the cause of
late blight of potatoes. As a result of the famine in Ireland caused by this disease, about 1 million people died and another 1.5 million emigrated (Alexopoulos,
et al. 1997). A number of other plant diseases are caused by species of
Phytophthora, including sudden oak death and ramorum blight caused by
P. ramorum, cacao black pod (P. megakarya), and many root rot diseases, such as
black shank of tobacco. Other members of the group include pathogens such as
Pythium aphanidermatum, the cause of
cottony blight of turf grasses;
Peronospora tabacina (tobacco blue mold);
Plasmopara viticola (downy mildew of grape);
Plasmopara halstedii (sunflower downy mildew); and many others. Finally, a group traditionally placed in the oomycetes is the Saprolegniales, or water molds, which cause diseases of fish and other aquatic vertebrates.
Characteristics of the Oomycota
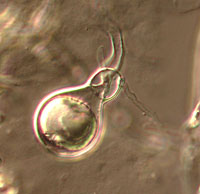
The Oomycota have long been considered fungi because they obtain their nutrients via absorption and many of them produce the filamentous threads known as mycelium characteristic of many fungi. The Oomycota now are classified as a distinct group based on a number of unique characteristics (Table 1). All members of the Oomycota undergo oogamous reproduction, meaning that diploid oospores are produced as zygotes following fertilization of haploid oospheres by haploid gametes. These oospores may be large and solitary (Figure 1) or smaller and numerous inside the oogonium. None of the true Fungi produce oospores.
Table 1. Major distinctions between the Oomycota in the Chromista and the true Fungi (Chytridiomycota, Glomeromycota, Zygomycota, Ascomycota, Basidiomycota)
| Character | Oomycota | True Fungi |
| Sexual reproduction | Heterogametangia. Fertilization of oospheres by nuclei from antheridia forming oospores. | Oospores not produced; sexual reproduction results in zygospores, ascospores or basidiospores |
| Nuclear state of vegetative mycelium | Diploid | Haploid or dikaryotic |
| Cell wall composition | Beta glucans, cellulose | Chitin. Cellulose rarely present |
| Type of flagella on zoospores, if produced | Heterokont, of two types, one whiplash, directed posteriorly, the other fibrous, ciliated, directed anteriorly | If flagellum produced, usually of only one type: posterior, whiplash |
| Mitochondria | With tubular cristae | With flattened cristae |
Another distinction is the cell wall composition. In the Oomycota, the cell walls are composed of beta glucans and cellulose rather than chitin as in the true Fungi.
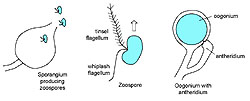
In addition the Oomycota produce motile zoospores with two kinds of flagella, one of which is a whiplash flagellum oriented posteriorly while the other is called a tinsel flagellum, which has a fibrous, ciliated structure and is oriented anteriorly (Figure 2). Behavior of zoospores of
Phytophthora is illustrated in a video at this
link. The occurrence of two kinds of flagella places these organisms in a group known as heterokonts. Although some true Fungi, namely the Chytridiomycota, produce stages with motile zoospores, their flagella are only of one kind, the posterior whiplash type.
 |
 |
|
Figure 1 |
Figure 2 |
A fourth major difference between the Oomycota and the true Fungi is that the vegetative cells of the Oomycota generally consist of coenocytic hyphae (hyphae without septa, i.e., without cross-walls), which contain diploid nuclei -- these organisms exist primarily in a
diploid state. This is unlike the true Fungi in which most of the mycelium is divided into cells by cross-walls, with each cell containing one, two, or more haploid nuclei. An exception are the Zygomycota, true Fungi with haploid nuclei but which produce coenocytic hyphae. Some members of the Oomycota produce unicellular thalli, a characteristic they share with some kinds of true Fungi.
Studies with the transmission electron microscope (TEM) in the late 1970s revealed differences in ultrastructural characteristics between the Oomycota and true Fungi. One distinguishing characteristic is that members of the Oomycota have mitochondria with tubular cristae and protoplasmic and nuclear-associated microtubules, while the true Fungi have flattened mitochondrial cristae. As more groups of organisms were examined using the TEM a relationship was hypothesized between the Oomycota and the heterokont algae (Table 1). This was the first modern evidence that the Oomycota should be regarded as “colorless” algae rather than true Fungi. Interestingly, as noted in Alexopoulos
et al., 1997, about 150 years ago Pringsheim (1858) suggested, in reference to the Saprolegniales [water molds], that the oomycetes “were allied with certain algae.”
Relationships of the Oomycota based on Molecular Results
As new tools for determining phylogenetic relationships were developed, especially those using molecular sequence data, they were applied to questions such as whether the Oomycota are more closely related to the heterokont algae or the true Fungi. Determining relationships using DNA sequence data is based on comparing sequence similarities in gene regions such as small or large subunits of the nuclear ribosomal DNA. Each base pair is regarded as a character and the sequence of base pairs is aligned such that analogous characters are compared. Mathematical algorithms running on powerful computers are used to analyze changes among the thousands of base pairs of gene regions. By determining how many changes have occurred between different groups of organisms, it is possible to estimate relative distance among groups and to develop an evolutionary tree that reflects those relationships.
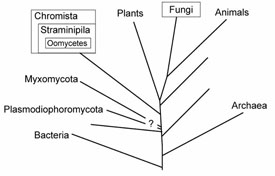
Results from a number of studies using molecular sequence data, combined with the ultrastructural similarities, confirm unequivocally that the Oomycota share a common ancestor with the other members of the heterokont algae or Chromista (Figure 3). In addition to the Oomycota, two smaller groups, the Hyphochytridiomycota and Labyrinthulomycota appear to be most closely related to the Chromista. The Chromista include several kinds of algae, namely the Phaeophyta or brown algae, Xanthophyta or yellow-green algae, Chrysophyta or golden algae, and Bacillariophyta or diatoms, as well as several smaller groups. The heterokont algae are distinctive among the algae in having the same two kinds of flagella as occur in the Oomycota, tubular mitochondrial cristae, and other ultrastructural similarities. The photosynthetic members of the Chromista possess chlorophyll c and other pigments that are not found in any other group. Some controversy still remains about exactly what to call this group of organisms. Most authors refer to the kingdom Chromista, phylum Heterokonta, while others place them in the kingdom Straminipila (sometimes written as Stramenopila, Patterson & Sogin, 1992).
 |
|
Figure 3 |
True Fungi or Eumycota
The true Fungi, or Eumycota, are now restricted to five major groups, each of which is regarded as a phylum in the Kingdom Fungi. One of these groups is the Chytridiomycota or chytrids. In some ways the chytrids are similar to the Saprolegniales in the Oomycota in that they are regarded as water molds. They feed on small-celled organisms and debris in aquatic environments. Another group of true Fungi is the Zygomycota or zygomycetes that include the bread molds (Mucorales). Their mostly non-septate mycelium gives them a superficial resemblance to the Oomycota. The arbuscular mycorrhizae were once regarded as part of the Zygomycota because they produce what appear to be zygospores. However, molecular data have shown that these root-associated fungi are quite distinct and should be regarded as their own phylum, the Glomeromycota. These fungi are ancient in origin, known from the fossil record to be associated with primitive land plants from the Devonian (Remy
et al., 1994).
The largest and most well known of the true Fungi are the Ascomycota or ascomycetes and the Basidiomycota or basidiomycetes. The Ascomycota include most of the lichenized fungi, yeast fungi, morels, cup fungi, and many kinds of microfungi such as pyrenomycetes and their asexual states, also referred to as mitotic or anamorphic states. In the past these have been variously referred to as asexual fungi, deuteromycetes or fungi imperfecti. These asexually reproducing ascomycetes include the hyphomycetes and coelomycetes many of which can now be placed among their relatives in the Ascomycota. The Basidiomycota or basidiomycetes include the rust and smut fungi most of which are obligate plant pathogens. Mushrooms, gasteromycetes, jelly fungi and polypores are also members of the Basidiomycota.
A recent noteworthy discovery is that the true Fungi are more closely related to animals than to plants (Baldauf & Palmer, 1993; Wainright
et al., 1993;
Figure 3), thus explaining why it is so difficult to develop antibiotics that are effective against human fungal pathogens without deleterious effects on humans.
The Remaining non-Fungi
A few other groups of organisms previously regarded as fungi are now known to belong outside the true Fungi (Figure 3). The Plasmodiophoromycota, including
Plasmodiophora brassicae, cause of cabbage club root, have proven difficult to place in the tree of life but they are not related to the true Fungi. Some evidence suggests that they too share a common ancestor with the Chromista but this is not conclusive. For some years it has been known that the Myxomycota or true slime molds, the Dictyosteliomycota or dictyostelid cellular slime molds, and the Acrasiomycota or acrasid cellular slime molds are not related to the true Fungi. Recent studies suggest that the Myxomycota represent an independent evolutionary lineage that diverged prior to the emergence of the “crown” groups of organisms that include the Fungi, Animalia, Plantae, Chromista, and Alveolates (Rhodophyta).
Conclusion
Most mycologists have not abandoned the study of the Oomycota and still define the organisms they study “as eukaryotic, heterotrophic osmotrophs in which assimilation takes place through a cell wall” (Dick, 1997). Adaptations of these organisms to obtaining their nutrients by absorption have resulted in considerable morphological convergence among them as exemplified by the similarity of the oomycetous white rusts to the uredinia of the true rust Fungi. Understanding the evolutionary relationships among these groups of organisms contributes greatly to our ability to develop strategies to control the diseases these organisms cause.
Literature Cited
Alexopoulos, C.J., C.W. Mims, and M. Blackwell. 1997. Introductory Mycology. John Wiley & Sons, Inc., New York, NY, USA.
Baldauf, S.L., and J.D. Palmer. 1993. Animals and fungi are each other's closest relatives: congruent evidence from multiple proteins. Proceedings of the National Academy of Sciences U.S.A. 90:11558-62.
Dick, M.W. 1997. Fungi, flagella and phylogeny. Mycological Research 101: 385-94.
Patterson, D.J., and M.L. Sogin. 1992. Eukaryote Origins and Protistan Diversity. Pp. 13-46. In: The Origin and Evolution of Prokaryotic and Eukaryotic Cells. Eds. H. Hartman and K. Matsuno. World Scientific, Singapore.
Pringsheim, N. 1858. Beiträge zur Morphologie und Systematik der Algen II. Die Saprolegnieen. Jahrbuch für Wissenschaftlichen Botanik 1:284-304.
Remy, W., T.N. Taylor, H. Hass, and H. Kerp. 1994. Four hundred-million-year-old vesicular arbuscular mycorrhizae. Proceedings of the National Academy of Sciences U.S.A. 91: 11841-3.
Wainright, P.O., G. Hinkle, M.L. Sogin, and S.K. Stickel. 1993. Monophyletic origins of the Metazoa: an evolutionary link with fungi. Science 260:340-2.
This publication is in the public domain and not copyrightable.
It may be freely reprinted with customary crediting of the source.
