 |
 |
 |
|
Figure 1 |
Figure 2 |
Figure 3 |
Several activities are associated with the conduct of this case study. Students will first examine the grass from the golf greens for symptoms/signs of fungal diseases to determine if this could be causing a reduction in the quality of the grass. After the diagnosis has been narrowed down to a possible nematode problem, students will conduct an extraction of nematodes from soil to determine the types and population density of nematodes present in the soil. Students must then determine if the nematodes identified could be responsible for the decrease in quality observed at the golf course. Finally, the students must recommend control measures that should be implemented to improve the quality of the grass.
This case was designed primarily to be used in a laboratory setting where dissecting and compound microscopes and centrifuges are available. It has been used as a part of an introductory plant pathology class with undergraduates majoring in plant pathology, horticulture, microbiology, crop sciences, agricultural education, and turfgrass management. In an undergraduate class the students will need assistance with the identification of the nematodes: illustrations of the important nematodes are provided. In a graduate class, students may be asked to identify nematode genera and make more determinations of what should be done related to nematode management.
CASE OBJECTIVES
The overall objectives of this case study are for students to become familiar with the methods for extracting and identifying nematodes from soil, symptoms associated with nematode infestations, and methods used for the management of nematode infestations. Specific objectives are:
- Observe symptoms of nematode infestation on grass species
- Extract nematodes from soil samples
- Obtain an understanding of how plant-parasitic as well as other nematodes are identified
- Obtain information on the importance of nematode control for the maintenance of golf course turfgrass
- Investigate options for nematode management, especially non-chemical control measures due to the decline in availability of nematicides
All objectives may not be accomplished in all classes depending on the level of student comprehension and availability of samples for study. Alternatively, for more advanced graduate level classes, additional objectives may be added with further development of the case.
CAST OF CHARACTERS
Bennett: Golf course manager at Sencopia Golf Course near Burton's Neck. He has been in the job approximately four months and is worried about keeping his job due to the poor appearance of some grass on the course
Gregory: Graduate student at Clemson University. His research focuses on the management of nematodes on golf courses through the use of non-chemical measures and the evaluation of new turfgrass varieties for resistance (reduced nematode reproduction) to important plant-parasitic nematodes.
THE CASE
Click here for the case
CLASSROOM MANAGEMENT
Case Summary
The collection, identification and management of nematodes on turfgrass are the foci of this case. Students will observe the symptoms and signs that can occur in turfgrass due to the presence of various nematode species. Activities also involve using different methods to extract nematodes from soil samples. Once nematodes are extracted, students identify the key characteristics that distinguish them from one another (used for identification to genera). Once the nematodes are identified, students must consider if the population density and type of nematodes present are the probable cause of the symptoms. If students determine that damaging nematode populations are present, they must propose possible measures to be incorporated into the golf course management system to improve the quality of the grass.
Suggestions on how to use this case
Various activities can be associated with this case depending on available resources. It was designed as a laboratory exercise. As currently written, students must have access to centrifuges as well as dissecting and compound microscopes for nematode identification. The case is provided to students as pre-reading material so they can become familiar with the case and nematode extraction procedures prior to the laboratory. This is important due to the numerous steps and procedures associated with the nematode extraction from soil. During the laboratory, students will extract nematodes from a soil sample and compare nematodes in the soil samples with those known to damage turfgrass. Students will also observe roots of the grass to determine if there are any possible fungal and bacterial problems associated with the roots. Root rots often occur when nematodes are causing extensive damage to roots. At the end of the laboratory, students should be able to determine if there are important plant-parasitic nematodes in the soil sample and if the levels of nematodes present could be causing the observed symptoms. Students will answer questions related to the case prior to the next laboratory meeting.
At the beginning of the next laboratory meeting, students will discuss their answers. They will determine, based on the identity and levels of nematodes, if the symptoms that Bennett is observing on the grass are caused by the presence of nematodes. The students will also discuss options for nematode management on established golf courses. A major point that students must consider is the fact that there are limited options available for successful nematode management. Students need to be aware that the best measure for nematode management is preventing the problem in the first place. "An ounce of prevention is worth a pound of cure."
Possible adaptations
These laboratory exercises can be conducted year-round using this case study especially in areas where golfing is a year-round sport. In areas with severe winter conditions, soil samples can be collected during the summer and placed in plastic bags and stored in a refrigerator for extraction later in the year. Nematodes survive well in moist soils in a refrigerator. In areas where grass samples are not available during the winter, pictures of grass and root symptoms may be used for the observation of symptoms and signs.
It is important for students to realize that nematodes present within a sample vary with climate and soil type and are managed in different ways. The important nematodes for the southeastern United States outlined for this case may be different from those significant in other regions. Nematodes are more prevalent in temperate to tropical regions of the world but they can cause problems anywhere. The management options acceptable for control in one area may not be available or legal for use in other areas. There are limited chemical options for nematode management. It is also important to remember that chemicals which are legally available for use in one area may not be available for legal use in another area. The chemicals that are legal today may not be legal for use next year as legal uses change. The case must be adapted to the area where it is being used and to the nematodes that are problems in that area. The case could be adapted to crops other than turfgrass. Various crops have nematode problems and these could be substituted in the case with changes made regarding the specific symptoms observed in the host plant. Examples of possible crops include soybeans, citrus, sugar beets, corn, cotton, peanuts, bananas and ornamental crops.
BACKGROUND INFORMATION
A general introduction to the plant-parasitic nematodes is featured at the APS Education website and provides excellent background information on nematodes (Lambert and Bekal 2002). This review covers nematode biology (including many pictures and figures), nematode survival, dissemination, plant nematode interactions (including descriptions of feeding types/strategies), nematode management, and lists many nematode related websites. This review, however, was not designed for hands-on work with nematodes and therefore does not include information on the extraction of nematodes from soil.
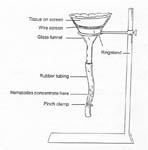
Two different methods can be used for the extraction of nematodes from soil depending on available resources. The centrifugal flotation method outlined in Procedure 1 (adapted from Jenkins, 1964) is a fast and efficient method for nematode extraction from soil but it requires access to a table top centrifuge and sieves for screening. If a centrifuge and sieves are not available, nematodes can be extracted using the Baermann funnel technique (Baermann 1917; Tylka and Jasalavich 2001, with a link to an illustrated Powerpoint file at the bottom of the page). This technique requires 1-3 days for extraction and only nematodes with motility in water will be extracted. This method does not extract sluggish forms of nematodes such as the ring nematodes, dead nematodes, or nematode eggs. The only equipment needed for the Baermann funnel technique (Procedure 2) are a ringstand, a filter funnel, a small piece of window screen, a tissue, and a length of rubber tubing with a clamp (Figure 4). Because of its simplicity, this extraction method can be done almost anywhere.
 |
|
Figure 4 |
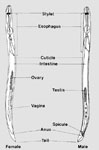
Plant-parasitic nematodes are often identified to genus based on their morphological characteristics (Figure 5). Students must transfer nematodes to slides in order to observe morphological characteristics using a compound microscope. This transfer can be accomplished with a thin wire. This is one of the hardest tasks for students to perform during the laboratory. Figure 5 illustrates the major morphological characters of female and male nematodes. The major nematodes known to affect turf worldwide are given in Table 1. Figures 6-20 illustrate the key nematode genera associated with turf along with some morphological characteristics used for their identification. The identification of nematodes to species level is not often needed to determine if nematodes are causing a problem on turf.
 |
|
Figure 5 |
Nematode thresholds (number/100 cm3 soil) are used to determine if nematodes are causing significant damage and if management options are needed. Table 1 includes a compilation of the average damage thresholds that have been used to determine management recommendations. These values were obtained from several references which are included with this case study (Couch 1995; Crow 2005; McCarty 2001). These thresholds vary widely depending on the variety of grass and on various environmental factors. For example, Bermuda, zoysia, centipede and seashore paspalum are somewhat tolerant of the stubby root nematodes (Paratrichodorus) with threshold of 150/100 cm3; however St. Augustine grass can be damaged at levels of only 40/100 cm3 (Crow 2001). These thresholds are based on conditions in the area where testing has been conducted and accurate thresholds are not available for many areas.
Procedure 1: Steps for centrifugal flotation extraction of nematodes from soil (Jenkins, 1964). This method is quick and will recover many forms of nematodes, including free-living and ectoparasitic nematodes, in high percentages.
-
Place 100 cm3 (approximately 1/2 cup) of soil into bucket and add water until approximately one-half full.
-
Mix thoroughly.
-
Let stand about 30 seconds.
-
Decant water through stacked 20 and 400 mesh sieves.
-
Wash residue on 400 mesh sieve with water into a centrifuge tube and spin for 4 minutes. Make sure to balance the tubes that are being put into the centrifuge.
-
Pour off the supernatant and discard.
-
Fill tubes with sugar solution. (one pound of cane sugar brought up to one quart with water or 450 g sugar made up to one liter with water)
-
Take a glass rod and mix up the pellet into the sugar solution.
-
Balance tubes by adding sugar solution as needed and then centrifuge for one minute.
-
Decant supernatant through a small 400 mesh sieve.
-
Rinse residue on sieve with water to remove sugar and to prevent injury to nematodes.
-
Using a wash bottle filled with water, rinse residue collected on the sieve into a vial and bring the volume up to 15 ml. Mix well and then transfer 1 ml of solution to a small Petri dish and observe nematodes using a dissecting microscope.
-
It may be necessary to transfer nematodes to glass slides with a thin wire and then use a compound microscope for identification to genera.
Look in various resources to determine the genera of plant parasitic nematodes associated with the sample (Table 1). Once nematodes have been identified, the number per 100 cc of soil should be determined. This is done by multiplying the number of nematodes of each genus in your 1-ml subsample by 15 (since you only counted 1/15th of the total sample).
Procedure 2: Baermann Funnel Technique. This technique is useful for extracting actively moving nematodes from soil or plant tissue. Nematodes move in thin films of water surrounding soil particles by pressing against soil particles and by using the surface tension of the water to propel themselves. Because most plant parasitic nematodes do not move fast enough to swim when they reach water filled pores, they will settle under the influence of gravity. The Baermann funnel utilizes this characteristic to cause active nematodes to move out of thin layers of soil and then sink in the water and become concentrated at the bottom of the funnel (Figure 4; Tylka and Jasalavich 2001, with a link to an illustrated Powerpoint file at the bottom of the page).
-
Attach a 8-10 cm length of rubber tubing to the stem of a glass funnel (10-15 cm in diameter) and attach the funnel to a ringstand.
-
Place a sturdy, fine mesh wire screen inside the funnel.
-
Close the open end of the tubing with a pinch clamp and fill the funnel with water so that the water just barely covers the screen.
-
Remove air bubbles trapped in the tubing either by tapping the tubing to allow bubbles to move to the top or by briefly opening the clamp to allow water and bubble to drain out.
-
Place 50 cm3 (1/4 cup) of sieved and mixed soil as a thin layer on top of a layer of paper tissue supported by the wire screen.
-
Carefully add water by lifting a corner of the tissue and pouring the water slowly against the inside surface of the funnel. Water should be added until the level is just above the screen to keep the soil moist.
-
Make sure that all edges and corners of the tissue are folded inside the funnel. This prevents siphoning of water out of the funnel.
-
Loosely cover the funnel with a Petri dish lid to reduce evaporation and leave the funnel undisturbed for 1-3 days. If needed, add more water to maintain the water level.
-
After 1-3 days, nematodes that have settled in the tubing can be collected by briefly opening the pinch clamp and allowing a small amount of water to drain into a small beaker or counting dish. Nematodes can be counted or identified immediately, or can be refrigerated in a covered container at 4-15 C.
-
Look in nematology texts and at the pictures provided (Table 1) to determine the types of nematodes present in the sample.
Table 1: Damage thresholds for various plant-parasitic nematodes of turfgrass (Couch 1995, Crow 2005, McCarty 2001)a.
|
Common name |
Nematode Genus |
Threshold for management (nematodes/100 cm3 soil)b |
Figure |
|
Awl |
Dolichodorus |
10-150 |
6
|
|
Cyst |
Heterodera |
10-40 |
7
|
|
Dagger |
Xiphinema |
150-300 |
8 |
|
Lance |
Hoplolaimus |
40-100 |
9 |
|
Needle |
Longidorus |
20 |
10 |
|
Pin |
Paratylenchus |
130 |
11 |
|
Ring |
Criconemoides |
150-1500 |
12 |
|
Root-knot |
Meloidogyne |
80-300 |
13
|
|
Lesion |
Pratylenchus |
150 |
14 |
|
Sheath |
Hemicycliophora |
80-200 |
15
|
|
Sheathoid |
Hemicriconemoides |
80-500 |
16 |
|
Spiral |
Helicotylenchus |
200-700 |
17 |
| |
Peltamigratus |
150 |
|
|
Sting |
Belonolaimus |
10-50 |
18 |
|
Stubby root |
Trichodorus |
50-100 |
19 |
| |
Paratrichodorus |
50-100 |
|
|
Stunt |
Tylenchorhynchus |
100-400 |
20 |
a Population thresholds are used for the justification of management.
b Variability in values is due to differences in the tolerance of various grass species. Values do not apply to all areas due to the variability of climates, soil types, and the interaction between plants and nematodes.
