Plant Health Progress - Plant Health Reviews - 22 June 2000
Accession DOI:10.1094/PHP-2000-0622-01-RV
Reproduced, with permission, from APSnet Features, 1999. The American Phytopathological Society, St. Paul, MN, U.S.A.
Robert W. Stack, Plant Pathology Dept. North Dakota State Univ.
Introduction
Fusarium head blight (FHB) of small grains (a.k.a."Scab") was first described just over a century ago and considered a major threat to wheat and barley during the early years of this century (6). In recent years FHB has again increased worldwide (28). The International Maize and Wheat Improvement Center (CIMMYT) has identified FHB as a major factor limiting wheat production in many parts of the world (7). During the past decade, several European conferences on Fusarium diseases have been dominated by reports on FHB of cereals. The most recent (1997) of these was attended by scientists from 28 countries on five continents (22). A 1996 conference on FHB sponsored by CIMMYT also had worldwide participation (7).
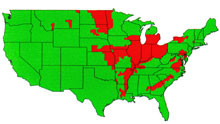
Fig. 1. Occurrence of FHB in the USA
1991-1996. |
|
Within the last dozen years there have been outbreaks of FHB in the Midwestern and Eastern states of the USA, as well as in Central and Eastern Canada. The most extended recent episode has been in the spring grain region of the upper Midwest, centered on the Red River Valley of North Dakota, Minnesota, and Manitoba that has experienced six consecutive years of disease. Losses have been large, and accumulated loss has brought ruin to many farmers in the region (18). The impact of FHB on the American wheat and barley industries in recent years has been sufficiently great to provoke a national response. A consortium of scientists and agribusiness leaders has developed a "US Wheat and Barley Scab Initiative" to target funding to solving this disease problem. Many information resources about FHB and current research on the disease can be accessed through their web site. The FHB situation in North America was reviewed in feature articles in Plant Disease in1994 (3) and again in 1997 (18). Aspects of FHB worldwide also have been reviewed (28, 7). The world literature on FHB is immense; fortunately, much of it can be accessed through the bibliographic web site maintained by the USDA Cereal Disease Lab in St.Paul, MN.
The Pathogen
Several species of the soil- and residue-borne fungus Fusarium are capable of inciting FHB. In parts of Northern Europe, for example, F. culmorum and F. avenaceum are the prevalent causes (8, 28). It is F. graminearum (FG), however, that has caused most of the recent outbreaks of FHB in the USA and Canada, as well as those in South America, Southern and Central Europe, China and Japan. In addition to small grain crops, FG has a wide host range among grasses. The name, in fact, means Fusarium "of the grasses". FG is also a superb colonizer of compromised or weakened plants of all sorts. FG has been recorded from dozens of crops and it is a particularly efficient colonizer of senescent maize stalks (42). Natural populations of F. graminearum and F. culmorum show considerable variability, but host specific strains have not been demonstrated (8, 21, 20).
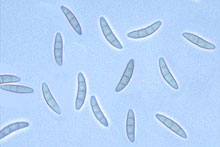
Fig. 2. Gibberella zeae ascospores.

Fig. 3. Gibberella zeae perithecia on maize kernels.
|
|
Most species of Fusarium are spread by dispersal of conidiospores that are blown or splashed to new infection courts. Among the species causing FHB, FG has an additional epidemiological advantage because it regularly and abundantly forms its sexual stage (Gibberella zeae). Ascospores produced by this stage are forcibly shot into the air, which greatly increases the ability of the fungus to disperse from colonized residue where perithecia form (14, 9).
Where G. zeae is the principal cause of FHB, ascospores are believed to be the principal primary inoculum in nature; however, both ascospores and conidiospores are effective as inoculum (33). Even if no FHB was present, the crop residue may be colonized after harvest providing carryover inoculum. Inoculation of field nurseries by spreading infested grain instead of naturally infested residue has been used by Chinese cereal breeders (39), and more recently in many North American screening nurseries testing for FHB resistance in wheat and barley. When maize is grown in the same region as small grains, as in the central United States, for example, senescent maize stalks are subject to massive colonization by FG. One Minnesota study found over 80% of the maize stalk residue was occupied by FG in the fall and that FG still controlled over 60% the following spring (42). This colonized residue provides a site for abundant sporulation during the next growing season and beyond (14). The saprobic part of the life cycle of F. graminearum is fueled by maize residue. Numerous observations, spanning six decades, indicate that when maize production moves into a small grain production area, occurrence of FG and the risk of FHB increase (15, 34, 27, 35). The mixture of maize, wheat and barley production, especially when combined with widespread use of reduced tillage and increased residue retention, sets the stage for major epidemics of FHB in small grains whenever weather favoring disease occurs during small grain heading and flowering (41).

Fig. 4. FHB Symptoms on barley heads.

Fig. 5. "Scabby" tombstone kernels of wheat.
|
|
Infection occurs when spores land on susceptible heads of wheat, rye, or barley. The flowering stage for wheat or rye and the newly emerged heads of barley (which flowers before heads emerge from the boot) are the most susceptible, but heads at later stages also can be infected.
The earliest infections generally kill the florets and no kernel develops at that point. Florets infected later may bear partially developed infected kernels, ("scabby" or "tombstone" kernels). Infections which occur after the kernels are filled may hardly affect kernel development, but such seemingly normal grains can harbor the fungus and may contain significant levels of trichothecene mycotoxins (4, 11, 32).
Epidemiology
There seems little doubt that the overriding limitation to FHB is moisture. Given a wet environment for an extended time, even a low inoculum level or a suboptimal temperature may not prevent FHB. The importance of extended wet periods favoring FHB was recognized early in the study of disease (29, 13), and such observations have been made many times since (18, 37). A succession of "wet cycle" years beginning in 1993 with much above average June and July rainfall has been linked to the protracted FHB epidemic in the Northern spring wheat region of the USA (17).
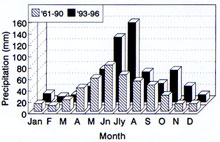
Fig 6. Comparison of high June-July rainfall in "scab years" in the spring wheat region. (click image for a larger view) |
|
Axel Anderson, using the extensive epidemiological resources at the US Army Biological Warfare Lab at Ft. Detrick, MD, was one of the first to quantify the relationship between temperature, moisture and FHB (1). It takes large differences in inoculum to produce measurable changes in FHB; one study found that a10-fold increase in inoculum caused only a two-fold increase in disease (33). Attempts to make use of the relationship between occurrence of moisture and plant development to forecast FHB have been made in the past (25) and more recently (24). Possible development of FHB forecasting is another research priority of the US Wheat and Barley Scab Initiative.
Chemical Control
Strong interest in use of fungicide sprays for control of FHB continues but the complete answer remains elusive. Timing and application are critical and additional products and technologies are needed (17). At present in North America, the best fungicide applications may provide a 50-60% reduction in FHB with a concomitant reduction in damaged kernels. Given present low commodity prices for cereal grains, this may not be an economic return in many cases (12). In Europe, with its different production cost structure for small grains, fungicide control of FHB has been studied more extensively (7). Fungicide applications may also reduce mycotoxin contamination in grain by 25-50% (5), but sometimes no fungicide treatment was at all effective (23). Improvement in FHB control by fungicide application is one of the priorities of the "US National Wheat and Barley Scab Initiative."
Resistance

Fig. 7. Inoculated wheat heads illustrating the expression of "Type II" resistance.
|
|
Resistance to FHB and its genetics has been the subject of two extensive recent reviews (21, 20). As far back as the 1920’s, plant pathologists and breeders observed that wheat genotypes seemed to differ in susceptibility to FHB, although it was difficult to clearly separate this from disease escape due to differences in maturity (13). Extensive research at the University of Minnesota extending from the 1920’s into the 1950’s laid the groundwork for much of our present knowledge about FHB resistance (10, 30). Unfortunately, this work was not continued, or we might be in a much better position to deal with this disease today (41).
Into the early 1960’s there was also major research on FHB in Japan but the effort given to wheat declined after that time (26). Japanese scientists have continued a strong program on FHB resistance in barley (36). Research on FHB resistance began in China in the 1950’s and has continued since (16). In Europe, research on FHB resistance has been active in several countries, notably Hungary, Poland, Austria, Germany, and the Netherlands (21, 20, 31).
When FHB resistance in wheat is discussed today, the topic of "Types of Resistance" is sure to arise. This concept was introduced by Schroeder and Christensen (30) and subsequently expanded by Mesterhazy (21). As the "Types" of resistance are described, they reflect the way each shows up in the plant. Perhaps "Expressions" or "Manifestations" of resistance would more correctly reflect the idea. Meidaner (20) used the term "Components of Resistance", but some plant breeders might feel that this could be confused with yield components. Whatever the objections to use of such terms, efforts at breeding for resistance probably have been sharpened by the distinctions so indicated. One example is the selective targeting of Type II resistance by the spikelet inoculation method (39, 38).
Following Mesterhazy’s (21) usage, the expressions of resistance are: I, resistance to primary infection; II, resistance to subsequent colonization after infection; III resistance expressed in the developing kernel; IV reduced accumulation of mycotoxins; V, yield tolerance. Other types also might exist. Evidence for expressions of types I, II, and III is quite strong and has been reported on numerous occasions as reviewed by Mesterhazy (21) and Meidaner (20). A good practical indicator of type I resistance is reduced incidence of infection. This needs to be determined in comparative trials using moderate airborne inoculum and under moderately favorable environmental conditions, since Type I resistance can be easily overwhelmed (30). Type II resistance has been the most studied in recent times. It is easy to detect, and good sources of it are well known. The single floret or single spikelet inoculation method selectively distinguishes this type of resistance (39). Type III, kernel resistance, manifests itself by a reduction in occurrence of Fusarium damaged kernels (tombstones). Each of these resistances is believed to be conditioned by multiple genes showing additive effects (20). The search for DNA markers ("quantitative trait loci" or QTL) for FHB resistance is actively underway in several laboratories, as two current papers show (2, 38).
Much less is known about resistance type IV, reduced mycotoxin accumulation. Some have challenged whether it even exists as a separate type or is just an expression of the effect of other factors, genetic or environmental. The work of Hart et al. (11), however, is suggestive in this regard. Resistance to mycotoxin accumulation in otherwise sound grain is a major goal in some barley breeding efforts (R. C. Horsley, barley breeder, North Dakota State Univ., Fargo, ND, personal communication 1999).
Yield tolerance is the ability of a plant to produce a crop even in the presence of disease at a level that would debilitate most other lines. Yield tolerance is well documented in some other plant disease systems, but evidence for it in FHB (as type V) is largely circumstantial, although a number of researchers believe it to occur (21).
The expression of resistance of each of these types may well involve several different physiological mechanisms, since multiple genes on different chromosomes are known (2, 38). In addition to these types of physiological resistance there are many other heritable traits which may directly or indirectly affect the plant's risk of exposure to Fusarium. Such traits as head type, phenology, trapped anthers, plant stature, rate of filling, to name just a few, have been implicated as influencing the level of FHB (20, 28).
The Future
While it may seem to some that little has changed since the 1920’s in regard to conquering FHB, we are on the threshold of major breakthroughs. Methods and technologies unheard of in those days are being used to solve this persistent disease problem. Highly active and even curative fungicides have been discovered, and application technologies are far more sophisticated than ever before. Molecular marker-assisted plant breeding for quantitatively inherited characters is already a reality, and is being extended to breeding for FHB resistance. In the nearer term, several wheat-breeding programs are ready to launch cultivars that are truly FHB resistant, developed by conventional breeding methods. There is every reason to expect that the history of FHB in the first decades of the 21st century will be quite different than anything in the 20th.
Literature Cited
1. Andersen, A. L. 1948. The Development of Gibberella zeae headblight of wheat. Phytopathology 38:595-611.
2. Bai, G., Kolb, F. L., Shaner, G., and Domier, L. L. 1999. Amplified fragment length polymorphism markers linked to a major quantitative trait locus controlling scab resistance in wheat. Phytopathology 89:343-348.
3. Bai, G. and Shaner, G. 1994. Scab of wheat: prospects for control. Plant Dis. 78:760-766.
4. Bechtel, D. B., Kaleikau, L. A., Gaines, R. L., and Seitz, L. M. 1985. The effects of Fusarium graminearum infection on wheat kernels. Cereal Chem. 62:191-197.
5. Boyacioglu, D., Hettiarachchy, N. S., and Stack, R. W. 1992. Effect of three systemic fungicides on deoxynivalenol (vomitoxin) production by Fusarium graminearum in wheat. Can. J. Plant Sci. 72:93-101.
6. Dickson, J.G., and Mains E.B. 1929. Scab of wheat and barley and its control. USDA Farmers’ Bulletin 1599. p. 1-18.
7. Dubin, H. J., Gilchrist, L., Reeves, J., and McNab, A., eds. 1997. Fusarium head scab: Global status and prospects. CIMMYT, Mexico, DF, Mexico. 130 p.
8. van Eeuwijk, F. A., Mesterhazy, A., Kling, C. I., Ruckenbauer, P., Saur, L., Burstmayr, H., Lemmens, M., Keizer, L. C. P., Maurin, N., and Snijders, C. H. A. 1995. Assessing non-specificity of resistance in wheat to head blight caused by inoculation with European strains of Fusarium culmorum, F. graminearum, and F. nivale using a multiplicative model for interaction. Theor. Appl. Genet. 90:221-228.
9. Fernando, W. G. D., Paulitz, T. C., Seaman, W. L., Dutilleul, P., and Miller, J.D. 1997. Head blight gradients caused by Gibberella zeae from area sources of inoculum in wheat field plots. Phytopathology 87:414-421.
10. Hanson, E. W., Ausemus, E. R., and Stakman, E. C. 1950. Varietal resistance of spring wheats to fusarial head blight. Phytopathology 40:902-914.
11. Hart, L. P., Pestka, J. J., and Liu, M. T. 1984. Effect of kernel development and wet periods on production of deoxynivalenol in wheat infected with Gibberella zeae. Phytopathology 74:1415-1418.
12. Hart, P. Ward, R., Bafus, R., and Bedford, K., compilers. 1998. Proceedings of the National Fusarium Head Blight Forum. Michigan State Univ., E. Lansing.
13. Immer, F. R., and Christensen, J. J. 1943. Studies on susceptibility of varieties and strains of barley to Fusarium and Helminthosporium kernel blight when tested under muslin tents or in nurseries. J. Am. Soc. Agron. 35:515-522.
14. Khonga, E. B., and Sutton, J. C. 1988. Inoculum production and survival of Gibberella zeae in maize and wheat residues. Can. J. Plant Pathol. 10: 232-239.
15.Koehler, B., Dickson, J. G., and Holbert, J, R. 1924. Wheat scab and corn rootrot caused by Gibberella saubinettii in relation to crop successions. J. Agr. Res. 27:861-883.
16. Liu, Z. Z., and Wang, Z. Y. 1990. Improved scab resistance in China: Sources of resistance and problems, p. 178-188. IN: D.A.Saunders, ed., Wheat for the Nontraditional Warm Areas. Proc. Int. Conf., CIMMYT, Mexico, D.F.
17. McMullen, M. P., Enz, J., Lukach, J., and Stover, R. 1997a. Environmental conditions associated with Fusarium head blight epidemics of wheat and barley in the Northern Great Plains, North America. Cereal Res. Commun. 25(3/2) 777-778.
18. McMullen, M. P., Jones, R., and Gallenberg, D. 1997b. Scab of wheat and barley: A re-emerging disease of devastating impact. Plant Dis. 81:1340-1348.
19. McMullen, M. P., Schatz, B., Stover, R., and Gregoire, T. 1997c. Studies of fungicide efficacy, application timing, and application technologies to reduce Fusarum head blight and deoxynivalenol. Cereal Res. Commun. 25(3/2) 779-780.
20. Meidaner, T. 1997. Breeding wheat and rye for resistance to Fusarium diseases. Plant Breeding 116:201-220.
21. Mesterhazy, A. 1995. Types and components of resistance to Fusarium head blight of wheat. Plant Breeding 114:377-386.
22. Mesterhazy, A., ed. 1997. Proc. Fifth European Fusarium Seminar. Cereal Res. Commun. 25(3):231-866.
23. Milus, E. A., and Parsons, C. E. 1994. Evaluation of foliar fungicides for controlling Fusarium head blight of wheat. Plant Dis. 78:697-699.
24. Moschini, R. C., and Fortugno, C. 1996. Predicting wheat head blight incidence using models based in meteorological factors in Pergamino, Argentina. Eur. J. Plant Pathol. 102:211-218.
25. Nakagawa, M., Watanabe, S., Gocho, H., and Nishio, K. 1965. Studies on the occurrence forecast of Gibberella zeae on wheat varieties caused by climatic factors. Bull. First Agron. Div., Tokai-Kinki National Agr. Expt. Sta 15:55-67 (in Japanese with English table legends, English summary p. 66-67).
26. Nisikado, Y. 1959. Studies on the wheat scab, caused by Gibberella zeae (Schw.) Petch and its control. Berichte d. Ohara Inst. Landw. Biol., Okayama Univ. 11(2):1-165 (English summary p. 141-165).
27. Obst, A., Lepschy-Von Gleissenthall, J., and Beck R. 1997. On the etiology of Fusarium head blight of wheat in south Germany - Preceding crops, weather conditions for inoculum production and head infection, proneness of the crop to infection and mycotoxin production. Cereal Res. Commun. 25 (3/2):699-703.
28. Parry, D. W., Jenkinson, P., and McLeod, L. 1995. Fusarium ear blight (scab) in small grains -- a review. Plant Pathol. 44:207-238.
29. Pugh, G. W., Johann, H., and Dickson, J. G. 1933. Factors affecting infection of wheat heads by Gibberella saubinetii. J. Agr. Res. 46:771-797.
30. Schroeder, H. W. and Christensen, J. J. 1963. Factors affecting resistance of wheat to scab caused by Gibberella zeae. Phytopathology 53:831-838.
31. Snijders, C. H. A. 1990. Genetic variation for resistance to Fusarium head blight in bread wheat. Euphytica 50:171-179.
32. Snijders, C. H. A., and Perkowski, J. 1990. Effects of head blight caused by Fusarium culmorum on toxin content and weight of wheat kernels. Phytopathology 80:566-570.
33. Stack, R. W. 1989. A comparison of the inoculum potential of ascospores and conidia of Gibberella zeae. Can. J. Plant Pathol. 11:137-142.
34. Strausbaugh, C. A., and Maloy, O. C. 1986. Fusarium scab of irrigated wheat in Central Washington. Plant Dis. 70:1104-1106.
35. Sutton, J. C. 1982. Epidemiology of wheat head blight and maize ear rot caused by Fusarium graminearum. Can. J. Plant Pathol. 4:195-209.
36. Takeda, K., and Heta, H. 1989. Establishing the testing method and a search for resistant varieties to Fusarium head blight in barley. Jap. J. Breeding 39:203-216.
37. Tuite, J., Shaner, G., and Everson, R.J. 1990. Wheat scab in soft red winter wheat in Indiana in 1986 and its relation to some quality measurements. Plant Dis. 74:959-962.
38. Waldron, B. L., Moreno-Sevilla, B., Anderson, J. A., Stack, R. W., and Frohberg, R. C. 1999. RFLP mapping of QTL for Fusarium head blight resistance in wheat. Crop Sci. 39:(3) (in press).
39. Wang, Y. Z., and Miller, J.D. 1988. Screening techniques and sources of resistance to Fusarium head blight, p 239-250. IN: A. R. Klatt, ed. Wheat Production Constraints in Tropical Environments. CIMMYT, Mexico, D. F.
40. Wilcoxson, R. D. 1993. Historical overview of scab research, p.1-5. IN: Proc. (1st) Regional Scab Forum, Moorhead, MN. Publ. Minn. Wheat Res. & Prom. Council, Red Lake Falls, MN 96 p.
41. Wilcoxson, R. D., Kommedahl, T., Ozmon, E. A., and Windels, C. E. 1988. Occurrence of Fusarium species in scabby wheat from Minnesota and their pathogenicity to wheat. Phytopathology 78:586-589.
42. Windels, C. E., and Kommedahl, T. 1984. Late-season colonization and survival of Fusarium graminearum group II in cornstalks in Minnesota. Plant Dis. 68:791-793.
